Single-Cell Multi-omics Assessment of Spinal Cord Injury Blocking via Cerium-doped Upconversion Antioxidant Nanoenzymes
- PMID: 39783786
- PMCID: PMC11848599
- DOI: 10.1002/advs.202412526
Single-Cell Multi-omics Assessment of Spinal Cord Injury Blocking via Cerium-doped Upconversion Antioxidant Nanoenzymes
Abstract
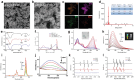
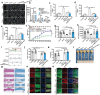
Spinal cord injury (SCI) impairs the central nervous system and induces the myelin-sheath-deterioration because of reactive oxygen species (ROS), further hindering the recovery of function. Herein, the simultaneously emergency treatment and dynamic luminescence severity assessment (SETLSA) strategy is designed for SCI based on cerium (Ce)-doped upconversion antioxidant nanoenzymes (Ce@UCNP-BCH). Ce@UCNP-BCH can not only efficiently eliminate the SCI localized ROS, but dynamically monitor the oxidative state in the SCI repair process using a ratiometric luminescence signal. Moreover, the classic basso mouse scale score and immunofluorescence analysis together exhibit that Ce@UCNP-BCH effectively facilitates the regeneration of spinal cord including myelin sheath, and promotes the functional recovery of SCI mice. Particularly, the study combines snATAC-eq and snRNA-seq to reveal the heterogeneity of spinal cord tissue following Ce@UCNP-BCH treatment. The findings reveal a significant increase in myelinating oligodendrocytes, as well as higher expression of myelination-related genes, and the study also reveals the gene regulatory dynamics of remyelination after treatment. Besides, the ETLSA strategy synergistically boosts ROS consumption through the superoxide dismutase (SOD)-related pathways after SOD-siRNA treatment. In conclusion, this SETLSA strategy with simultaneously blocking and dynamic monitoring oxidative stress has enriched the toolkit for promoting SCI repair.
Keywords: cerium‐doped nanoenzymes; myelination; oxidative stress monitoring; reactive oxygen species (ROS); spinal cord injury (SCI).
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Courtine G., Sofroniew M. V., Nat. Med. 2019, 25, 898. - PubMed
-
- a) Li Z., Zhao T., Ding J., Gu H., Wang Q., Wang Y., Zhang D., Gao C., Bioact. Mater. 2023, 19, 550; - PMC - PubMed
- b) Zuo Y., Ye J., Cai W., Guo B., Chen X., Lin L., Jin S., Zheng H., Fang A., Qian X., Abdelrahman Z., Wang Z., Zhang Z., Chen Z., Yu B., Gu X., Wang X., Nat. Nanotechnol. 2023, 18, 1230. - PubMed
-
- a) Xiong T., Yang K., Zhao T., Zhao H., Gao X., You Z., Fan C., Kang X., Yang W., Zhuang Y., Chen Y., Dai J., Adv. Sci. 2023, 10, e2205997; - PMC - PubMed
- b) Li L., Guo J., Wang Y., Xiong X., Tao H., Li J., Jia Y., Hu H., Zhang J., Adv. Sci. 2018, 5, 1800781; - PMC - PubMed
- c) Zhang Z., Guan J., Jiang Z., Yang Y., Liu J., Hua W., Mao Y., Li C., Lu W., Qian J., Zhan C., Nat. Commun. 2019, 10, 3561. - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 82102314/National Natural Science Foundation of China
- 82372504/National Natural Science Foundation of China
- 32170977/National Natural Science Foundation of China
- 82272248/National Natural Science Foundation of China
- 82102444/National Natural Science Foundation of China
- 2022A1515010438/Guangdong Basic and Applied Basic Research Foundation
- 2024A1515010268/Guangdong Basic and Applied Basic Research Foundation
- JNU1AF- CFTP- 2022- a01206/Clinical Frontier Technology Program of the First Affiliated Hospital of Jinan University, China
- 2023A04J1284/Guangzhou Science and Technology Plan Project
- 202201020018/Guangzhou Science and Technology Plan Project
- 2023A03J1024/Guangzhou Science and Technology Plan Project
- QT-2024-039/Young Talent Support Project of Guangzhou Association for Science and Technology
- 82322042/National Science Foundation for Excellent Young Project
- 2021YFC2302200/National Key Research and Development Program
- 22050000282/Natural Science Fund of Guangdong Province for Distinguished Young Scholars
- ZNSA-2021012/Zhongnanshan Medical Foundation of Guangdong Province
- SL2024A04J01463/Basic and Applied Basic Research Topic of Guangzhou Sailing Project for Young Doctor
LinkOut - more resources
Full Text Sources
Medical
