CT-P39 Compared With Reference Omalizumab in Chronic Spontaneous Urticaria: Results From a Double-Blind, Randomized, Active-Controlled, Phase 3 Study
- PMID: 39785096
- PMCID: PMC12368742
- DOI: 10.1111/all.16446
CT-P39 Compared With Reference Omalizumab in Chronic Spontaneous Urticaria: Results From a Double-Blind, Randomized, Active-Controlled, Phase 3 Study
Abstract
Background: This study compared the therapeutic equivalence of CT-P39 (an omalizumab biosimilar) and EU-approved reference omalizumab (ref-OMA) in patients with chronic spontaneous urticaria.
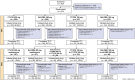
Methods: This double-blind, randomized, active-controlled Phase 3 study (NCT04426890) included two 12-week treatment periods (TPs). In TP1, patients received CT-P39 300 mg, ref-OMA 300 mg, CT-P39 150 mg, or ref-OMA 150 mg. In TP2, patients treated with ref-OMA 300 mg were rerandomized to CT-P39 300 mg or ref-OMA 300 mg; patients initially randomized to CT-P39 300 mg continued this regimen; and patients initially randomized to CT-P39 or ref-OMA 150 mg received 300 mg dosing with the same drug. The primary endpoint for the assessment of therapeutic equivalence of CT-P39 300 mg and ref-OMA 300 mg was change from baseline in weekly itch severity score (ISS7) at week 12.
Results: In TP1, 619 patients were randomized (CT-P39 300 mg, n = 204; ref-OMA 300 mg, n = 205; CT-P39 150 mg, n = 107; ref-OMA 150 mg, n = 103). Equivalence was demonstrated between CT-P39 300 mg and ref-OMA 300 mg for mean change from baseline in ISS7 at week 12; confidence intervals (CIs) were within predefined equivalence margins: global analysis: treatment difference 0.77, 95% CI -0.37 to 1.90; US analysis: treatment difference 0.70, 90% CI -0.22 to 1.63. The proportion of patients experiencing ≥ 1 treatment-related adverse event was comparable across groups. Secondary efficacy, quality of life, pharmacokinetic, safety, and immunogenicity outcomes were comparable between groups at a given dose level, with no evident impact of switching.
Conclusions: Equivalent efficacy was observed between CT-P39 and ref-OMA, with comparable safety also evident.
Keywords: CT‐P39; anti‐IgE; biosimilar; chronic spontaneous urticaria; omalizumab.
© 2025 The Author(s). Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
S.S.S. reports grant, research, and/or clinical trial support from Allakos, Amgen, Escient, National Institutes of Health, Novartis, Regeneron, and Sanofi and has served as a consultant or advisory board member for Allakos, Aquestive, Celltrion, Escient, Granular Therapeutics, Innate, Novartis, Regeneron, and Sanofi. M.M. was a speaker and/or advisor for, and/or has received research funding from, Allakos, Alexion, Almirall, Alvotech, Amgen, Aquestive, argenx, AstraZeneca, Celldex, Celltrion, Clinuvel, Escient, Evommune, Excellergy, GSK, Incyte, Jasper, Kashiv, Kyowa Kirin, Leo Pharma, Lilly, Menarini, Mitsubishi Tanabe Pharma, Moxie, Noucor, Novartis, Orion Biotechnology, Resonance Medicine, Sanofi/Regeneron, Santa Ana Bio, Septerna, Servier, Third Harmonic Bio, ValenzaBio, Vitalli Bio, Yuhan Corporation, and Zura Bio. A.R. is or recently was a speaker and/or advisor for, and/or has received research funding from, Almirall, Alumis, Alvotech, Amgen, AnaptysBio, argenx, AstraZeneca, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celltrion, Chema Rzeszów, Galderma, Horizon, Incyte, InflaRx, Janssen, Kiniksa, Leo Pharma, Lilly, Novartis, Numab, Pfizer, Sanofi/Regeneron, Takeda, Trevi Therapeutics, and UCB. S.K., K.A., S.K., S.L., J.Ka, and J.Kim are employees of the study sponsor Celltrion. C.G. has served as a consultant or advisory board member for argenx, Blueprint Medicines, Celltrion, Novartis, and Sanofi; sits on steering committees for AB Science (without payment) and Blueprint Medicines; and has previously chaired a Data Safety Monitoring Board for CSL Behring. Y.D., E.S., M.R., B.K., C.W.P., G.P., and M.C. declare no conflicts of interest.
Figures

References
-
- Kaplan A., Lebwohl M., Giménez‐Arnau A. M., Hide M., Armstrong A. W., and Maurer M., “Chronic Spontaneous Urticaria: Focus on Pathophysiology to Unlock Treatment Advances,” Allergy 78, no. 2 (2023): 389–401. - PubMed
-
- Kolkhir P., Church M. K., Weller K., Metz M., Schmetzer O., and Maurer M., “Autoimmune Chronic Spontaneous Urticaria: What We Know and What We Do Not Know,” Journal of Allergy and Clinical Immunology 139, no. 6 (2017): 1772–1781.e1. - PubMed
-
- Kolkhir P., Muñoz M., Asero R., et al., “Autoimmune chronic spontaneous urticaria,” Journal of Allergy and Clinical Immunology 149, no. 6 (2022): 1819–1831. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

