TBK1 Targeting Is Identified as a Therapeutic Strategy to Enhance CAR T-Cell Efficacy Using Patient-Derived Organotypic Tumor Spheroids
- PMID: 39785827
- PMCID: PMC11790382
- DOI: 10.1158/2326-6066.CIR-23-1011
TBK1 Targeting Is Identified as a Therapeutic Strategy to Enhance CAR T-Cell Efficacy Using Patient-Derived Organotypic Tumor Spheroids
Abstract
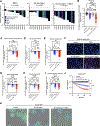
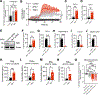
Novel therapeutic strategies are needed to improve the efficacy of chimeric antigen receptor (CAR) T cells as a treatment of solid tumors. Multiple tumor microenvironmental factors are thought to contribute to resistance to CAR T-cell therapy in solid tumors, and appropriate model systems to identify and examine these factors using clinically relevant biospecimens are limited. In this study, we examined the activity of B7-H3-directed CAR T cells (B7-H3.CAR-T) using 3D microfluidic cultures of patient-derived organotypic tumor spheroids (PDOTS) and then confirmed the activity of B7-H3.CAR T cells in PDOTS. Although B7-H3 expression in PDOTS was associated with B7-H3.CAR-T sensitivity, mechanistic studies revealed dynamic upregulation of co-inhibitory receptors on CAR T-cells following target cell encounter that led to CAR T-cell dysfunction and limited efficacy against B7-H3-expressing tumors. PD-1 blockade restored CAR T-cell activity in monotypic and organotypic tumor spheroids with improved tumor control and upregulation of effector cytokines. Given the emerging role of TANK-binding kinase 1 (TBK1) as an immune evasion gene, we examined the effect of TBK1 inhibition on CAR T-cell efficacy. Similar to PD-1 blockade, TBK1 inhibition restored CAR T-cell activity in monotypic and organotypic tumor spheroids, prevented CAR T-cell dysfunction, and enhanced CAR T-cell proliferation. Inhibition or deletion of TBK1 also enhanced the sensitivity of cancer cells to immune-mediated killing. Taken together, our results demonstrate the feasibility and utility of ex vivo profiling of CAR T cells using PDOTS and suggest that targeting TBK1 could be used to enhance CAR T-cell efficacy by overcoming tumor-intrinsic and -extrinsic resistance mechanisms.
©2025 American Association for Cancer Research.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
R.W.J. is a member of the advisory board for and has a financial interest in Xsphera Biosciences Inc., a company focused on using
Figures





References
-
- Del Bufalo F, De Angelis B, Caruana I, Del Baldo G, De Ioris MA, Serra A, et al. GD2-CART01 for Relapsed or Refractory High-Risk Neuroblastoma. N Engl J Med. 2023;388:1284–95. - PubMed
MeSH terms
Substances
Grants and funding
- K08 CA226391/CA/NCI NIH HHS/United States
- R37 CA283560/CA/NCI NIH HHS/United States
- R01 CA226981/CA/NCI NIH HHS/United States
- P50CA127003/National Institutes of Health (NIH)
- R01 DE028172/DE/NIDCR NIH HHS/United States
- 1R37CA283560-01/National Cancer Institute (NCI)
- Cholangiocarcinoma Foundation
- Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (AMRF)
- P50 CA127003/CA/NCI NIH HHS/United States
- W81XWH2110433/U.S. Department of Defense (DOD)
- R01 CA280085/CA/NCI NIH HHS/United States
- R01DE028172/National Institutes of Health (NIH)
- R03 CA280302/CA/NCI NIH HHS/United States
- K08CA226391/National Cancer Institute (NCI)
- R03CA280302/National Institutes of Health (NIH)
- TargetCancer Foundation
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

