CO Cryo-Sorption as a Surface-Sensitive Spectroscopic Probe of the Active Site Density of Single-Atom Catalysts
- PMID: 39786323
- PMCID: PMC11878345
- DOI: 10.1002/anie.202420673
CO Cryo-Sorption as a Surface-Sensitive Spectroscopic Probe of the Active Site Density of Single-Atom Catalysts
Abstract
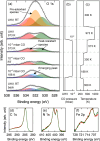
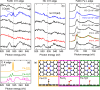
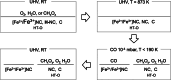
Quantifying the number of active sites is a crucial aspect in the performance evaluation of single metal-atom electrocatalysts. A possible realization is using adsorbing gas molecules that selectively bind to the single-atom transition metal and then probing their surface density using spectroscopic tools. Herein, using in situ X-ray photoelectron spectroscopy (XPS) and near edge X-ray absorption fine structure (NEXAFS) spectroscopy, we detect adsorbed CO gas molecules on a FeNC oxygen reduction single atom catalyst. Correlating XPS and NEXAFS, we develop a simple surface- and chemically-sensitive protocol to accurately and quickly quantify the active site density. Density functional theory-based X-ray spectra simulations reaffirm the assignment of the spectroscopic fingerprints of the CO molecules adsorbed at Fe-N4-C sites, and provide additional unexpected structural insights about the active site needed to explain the low-temperature CO adsorption. Our work represents an important step towards an accurate quantitative catalytic performance evaluation, and thus towards developing reliable material design principles and catalysts.
Keywords: ab initio calculations; adsorption; density functional calculations; heterogeneous catalysis; photoelectron spectroscopy; x-ray absorption spectroscopy.
© 2025 The Author(s). Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
There is no conflict of interest to declare.
Figures



References
Grants and funding
- 2022R1F1A1064235/National Research Foundation of Korea (Ministry of Science and Technology)
- 2021R1C1C1008776/National Research Foundation of Korea (Ministry of Science and Technology)
- 2023R1A2C2005278/National Research Foundation of Korea (Ministry of Science and Technology)
- A426220/Korea Basic Science Institute
- MR/X023109/1/UKRI Future Leaders Fellowship
LinkOut - more resources
Full Text Sources

