Abstraction of Hydride from Alkanes and Dihydrogen by the Perfluorotrityl Cation
- PMID: 39786341
- PMCID: PMC11976196
- DOI: 10.1002/anie.202422190
Abstraction of Hydride from Alkanes and Dihydrogen by the Perfluorotrityl Cation
Abstract
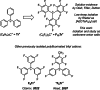
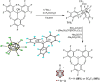
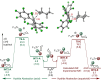
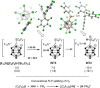
Lewis acids play a central role in a large variety of chemical transformations. The reactivity of the strongest Lewis acids is typically studied in the context of affinity towards hard bases, such as fluoride or oxygenous species. Carbocations can be viewed as soft Lewis acids, possessing significant affinity for softer bases, such as hydride. This work presents the ambient-temperature isolation of salts of the perfluorotrityl cation ((C6F5)3C+ or F15Tr+) in combination with halogenated carborane anions. The F15Tr+ cation exhibits remarkable hydride affinity, illustrated by the observation of hydride abstraction from dihydrogen, and of the rapid abstraction of hydride from -CH2-groups in alkanes. Theoretical studies support the favorability of hydride abstraction from dihydrogen, and indicate that the hydride abstraction from alkanes proceeds via a concerted hydride transfer process that is sensitive to steric effects.
Keywords: Carbocation; Carborane; Hydride abstraction; Silylium.
© 2025 The Author(s). Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Olah G. A., Prakash G. K. S., Carbocation Chemistry. John Wiley & Sons, Inc., 2004.
-
- Chen E. Y.-X., Marks T. J., Chem. Rev. 2000, 100, 1391–1432. - PubMed
-
- Kim K.-C., Reed C. A., Long G. S., Sen A., J. Am. Chem. Soc. 2002, 124, 7662–7663. - PubMed
-
- Mehlmann P., Witteler T., Wilm L. F. B., Dielmann F., Nat. Chem. 2019, 11, 1139–1143. - PubMed
-
- Kim K.-C., Reed C. A., Elliott D. W., Mueller L. J., Tham F., Lin L., Lambert J. B., Science 2002, 297, 825–827. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

