The effect of transcutaneous auricular vagus nerve stimulation on cardiovascular function in subarachnoid hemorrhage patients: A randomized trial
- PMID: 39786346
- PMCID: PMC11717364
- DOI: 10.7554/eLife.100088
The effect of transcutaneous auricular vagus nerve stimulation on cardiovascular function in subarachnoid hemorrhage patients: A randomized trial
Abstract
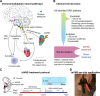
Background: Subarachnoid hemorrhage (SAH) is characterized by intense central inflammation, leading to substantial post-hemorrhagic complications such as vasospasm and delayed cerebral ischemia. Given the anti-inflammatory effect of transcutaneous auricular vagus nerve stimulation (taVNS) and its ability to promote brain plasticity, taVNS has emerged as a promising therapeutic option for SAH patients. However, the effects of taVNS on cardiovascular dynamics in critically ill patients, like those with SAH, have not yet been investigated. Given the association between cardiac complications and elevated risk of poor clinical outcomes after SAH, it is essential to characterize the cardiovascular effects of taVNS to ensure this approach is safe in this fragile population. Therefore, this study assessed the impact of both acute and repetitive taVNS on cardiovascular function.
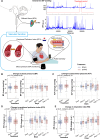
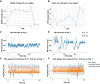
Methods: In this randomized clinical trial, 24 SAH patients were assigned to either a taVNS treatment or a sham treatment group. During their stay in the intensive care unit, we monitored patient electrocardiogram readings and vital signs. We compared long-term changes in heart rate, heart rate variability (HRV), QT interval, and blood pressure between the two groups. Additionally, we assessed the effects of acute taVNS by comparing cardiovascular metrics before, during, and after the intervention. We also explored acute cardiovascular biomarkers in patients exhibiting clinical improvement.
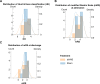
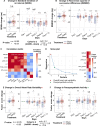
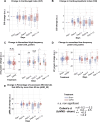
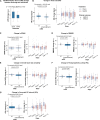
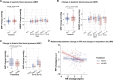
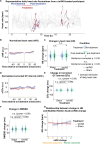
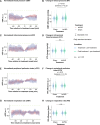
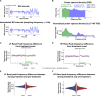
Results: We found that repetitive taVNS did not significantly alter heart rate, QT interval, blood pressure, or intracranial pressure (ICP). However, repetitive taVNS increased overall HRV and parasympathetic activity compared to the sham treatment. The increase in parasympathetic activity was most pronounced from 2 to 4 days after initial treatment (Cohen's d = 0.50). Acutely, taVNS increased heart rate, blood pressure, and peripheral perfusion index without affecting the corrected QT interval, ICP, or HRV. The acute post-treatment elevation in heart rate was more pronounced in patients who experienced a decrease of more than one point in their modified Rankin Score at the time of discharge.
Conclusions: Our study found that taVNS treatment did not induce adverse cardiovascular effects, such as bradycardia or QT prolongation, supporting its development as a safe immunomodulatory treatment approach for SAH patients. The observed acute increase in heart rate after taVNS treatment may serve as a biomarker for SAH patients who could derive greater benefit from this treatment.
Funding: The American Association of Neurological Surgeons (ALH), The Aneurysm and AVM Foundation (ALH), The National Institutes of Health R01-EB026439, P41-EB018783, U24-NS109103, R21-NS128307 (ECL, PB), McDonnell Center for Systems Neuroscience (ECL, PB), and Fondazione Neurone (PB).
Clinical trial number: NCT04557618.
Keywords: autonomic balance; cardiovascular; heart rate variability; human; medicine; neuroscience; subarachnoid hemorrhage; transcutaneous auricular vagus nerve stimulation.
© 2024, Tan, Huguenard et al.
Conflict of interest statement
GT, KD, PD, XL, ZL, MA, KL, AV, TK, JO, GZ, PB No competing interests declared, AH Has stock ownership in Aurenar, EL Has stock ownership in Neurolutions, Face to Face Biometrics, Caeli Vascular, Acera, Sora Neuroscience, Inner Cosmos, Kinetrix, NeuroDev, Inflexion Vascular, Aurenar, Cordance Medical, Silent Surgical, and Petal Surgical; consultant for E15, Neurolutions, Inc, Petal Surgical; Washington University owns equity in Neurolutions
Figures





Update of
-
The effect of transcutaneous auricular vagus nerve stimulation on cardiovascular function in subarachnoid hemorrhage patients: a safety study.medRxiv [Preprint]. 2024 Sep 8:2024.04.03.24304759. doi: 10.1101/2024.04.03.24304759. medRxiv. 2024. Update in: Elife. 2025 Jan 09;13:RP100088. doi: 10.7554/eLife.100088. PMID: 38633771 Free PMC article. Updated. Preprint.
Similar articles
-
The effect of transcutaneous auricular vagus nerve stimulation on cardiovascular function in subarachnoid hemorrhage patients: a safety study.medRxiv [Preprint]. 2024 Sep 8:2024.04.03.24304759. doi: 10.1101/2024.04.03.24304759. medRxiv. 2024. Update in: Elife. 2025 Jan 09;13:RP100088. doi: 10.7554/eLife.100088. PMID: 38633771 Free PMC article. Updated. Preprint.
-
Non-invasive Auricular Vagus nerve stimulation for Subarachnoid Hemorrhage (NAVSaH): Protocol for a prospective, triple-blinded, randomized controlled trial.PLoS One. 2024 Aug 23;19(8):e0301154. doi: 10.1371/journal.pone.0301154. eCollection 2024. PLoS One. 2024. PMID: 39178291 Free PMC article.
-
Non-invasive Auricular Vagus nerve stimulation for Subarachnoid Hemorrhage (NAVSaH): Protocol for a prospective, triple-blinded, randomized controlled trial.medRxiv [Preprint]. 2024 Mar 19:2024.03.18.24304239. doi: 10.1101/2024.03.18.24304239. medRxiv. 2024. Update in: PLoS One. 2024 Aug 23;19(8):e0301154. doi: 10.1371/journal.pone.0301154. PMID: 38562875 Free PMC article. Updated. Preprint.
-
Does transcutaneous auricular vagus nerve stimulation affect vagally mediated heart rate variability? A living and interactive Bayesian meta-analysis.Psychophysiology. 2021 Nov;58(11):e13933. doi: 10.1111/psyp.13933. Epub 2021 Sep 2. Psychophysiology. 2021. PMID: 34473846 Review.
-
Clinical application of transcutaneous auricular vagus nerve stimulation: a scoping review.Disabil Rehabil. 2024 Dec;46(24):5730-5760. doi: 10.1080/09638288.2024.2313123. Epub 2024 Feb 16. Disabil Rehabil. 2024. PMID: 38362860
Cited by
-
Transcutaneous Auricular Vagus Nerve Stimulation Reduces Inflammatory Biomarkers and May Improve Outcomes after Large Vessel Occlusion Strokes: Results of the Randomized Clinical Trial NUVISTA.medRxiv [Preprint]. 2025 Mar 7:2025.03.06.25323500. doi: 10.1101/2025.03.06.25323500. medRxiv. 2025. PMID: 40093228 Free PMC article. Preprint.
-
Auricular vagus nerve stimulation for mitigation of inflammation and vasospasm in subarachnoid hemorrhage: a single-institution randomized controlled trial.J Neurosurg. 2025 Jan 24;142(6):1720-1731. doi: 10.3171/2024.10.JNS241643. Print 2025 Jun 1. J Neurosurg. 2025. PMID: 39854697 Free PMC article. Clinical Trial.
References
-
- Addorisio ME, Imperato GH, de Vos AF, Forti S, Goldstein RS, Pavlov VA, van der Poll T, Yang H, Diamond B, Tracey KJ, Chavan SS. Investigational treatment of rheumatoid arthritis with a vibrotactile device applied to the external ear. Bioelectronic Medicine. 2019;5:4. doi: 10.1186/s42234-019-0020-4. - DOI - PMC - PubMed
-
- Bazett HC. An analysis of the time-relations of electrocardiograms. Heart (British Cardiac Society) 1920;7:353–370.
-
- Bjerkne Wenneberg S, Löwhagen Hendén PM, Oras J, Naredi S, Block L, Ljungqvist J, Odenstedt Hergès H. Heart rate variability monitoring for the detection of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Acta Anaesthesiologica Scandinavica. 2020;64:945–952. doi: 10.1111/aas.13582. - DOI - PubMed
-
- Bonaz B, Sinniger V, Hoffmann D, Clarençon D, Mathieu N, Dantzer C, Vercueil L, Picq C, Trocmé C, Faure P, Cracowski J, Pellissier S. Chronic vagus nerve stimulation in Crohn’s disease: a 6‐month follow‐up pilot study. Neurogastroenterology & Motility. 2016;28:948–953. doi: 10.1111/nmo.12792. - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

