OBI-992, a Novel TROP2-Targeted Antibody-Drug Conjugate, Demonstrates Antitumor Activity in Multiple Cancer Models
- PMID: 39786401
- PMCID: PMC11791482
- DOI: 10.1158/1535-7163.MCT-24-0588
OBI-992, a Novel TROP2-Targeted Antibody-Drug Conjugate, Demonstrates Antitumor Activity in Multiple Cancer Models
Abstract
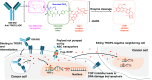
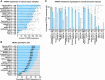
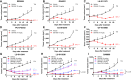
Trophoblast cell surface antigen 2 (TROP2) is highly expressed in multiple cancers relative to normal tissues, supporting its role as a target for cancer therapy. OBI-992 is an antibody-drug conjugate (ADC) derived from a novel TROP2-targeted antibody linked to the topoisomerase 1 (TOP1) inhibitor exatecan via an enzyme-cleavable hydrophilic linker, with a drug-antibody ratio of 4. This study evaluated and compared the antitumor activity of OBI-992 with that of benchmark TROP2-targeted ADCs datopotamab deruxtecan (Dato-DXd) and sacituzumab govitecan (SG) in cell line-derived xenograft (CDX) and patient-derived xenograft (PDX) models. OBI-992 treatment exhibited statistically significant antitumor activity versus controls at doses of 3 and 10 mg/kg in various CDX and PDX models, demonstrating comparable or better antitumor activity with benchmark ADCs. In a large-tumor model, longer survival times were observed in OBI-992-treated mice compared with Dato-DXd-treated mice. OBI-992 treatment induced marked bystander killing of TROP2-negative cells in the presence of nearby TROP2-positive cells in both in vitro and in vivo studies. In lung adenocarcinoma CDX models with overexpression of either P-glycoprotein (P-gp) or breast cancer resistance protein (BCRP) to mimic ATP-binding cassette transporter-mediated multidrug resistance, OBI-992 treatment maintained antitumor activity when Dato-DXd treatment became less effective. The combination of OBI-992 at suboptimal doses with either poly (ADP-ribose) polymerase (PARP) inhibitors or an immune check point inhibitor produced synergistic antitumor effects in mouse models. Taken together, these translational results support further development of OBI-992 as a cancer therapy.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
W.-F. Li reports a patent for PCT/US2024/021729 pending and a patent 113111546 pending. T.-Y. Huang reports a patent for PCT/US2024/021729 pending and a patent 113111546 pending. M.-T. Lai reports a patent for PCT/US2024/021729 pending and a patent for 113111546/Taiwan pending. No disclosures were reported by the other authors.
Figures




References
-
- Shastry M, Gupta A, Chandarlapaty S, Young M, Powles T, Hamilton E. Rise of antibody-drug conjugates: the present and future. Am Soc Clin Oncol Educ Book 2023;43:e390094. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

