A hitchhiker's guide to cerebrospinal fluid biomarkers for neuro-oncology
- PMID: 39786477
- PMCID: PMC12187377
- DOI: 10.1093/neuonc/noae276
A hitchhiker's guide to cerebrospinal fluid biomarkers for neuro-oncology
Erratum in
-
Erratum to: A hitchhiker's guide to cerebrospinal fluid biomarkers for neuro-oncology.Neuro Oncol. 2025 Jul 1:noaf148. doi: 10.1093/neuonc/noaf148. Online ahead of print. Neuro Oncol. 2025. PMID: 40591417 No abstract available.
Abstract
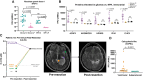
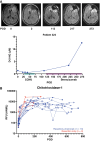
Cerebrospinal fluid (CSF) has emerged as a valuable liquid biopsy source for glioma biomarker discovery and validation. CSF produced within the ventricles circulates through the subarachnoid space, where the composition of glioma-derived analytes is influenced by the proximity and anatomical location of sampling relative to tumor, in addition to underlying tumor biology. The substantial gradients observed between lumbar and intracranial CSF compartments for tumor-derived analytes underscore the importance of sampling site selection. Moreover, radiographic features, such as tumor-CSF contact and blood-brain barrier disruption, are critical covariates that may affect biomarker detection and the abundance of plasma-derived analytes in CSF, respectively. Longitudinal intracranial CSF sampling, enabled by access devices like Ommaya reservoirs, may offer a window into treatment response and disease progression, though variability in analyte yield, sample volumes, and the dynamic effects of surgical resection pose challenges. This review critically evaluates the anatomic, radiographic, and longitudinal factors, or "time-space continuum," that impact glioma CSF biomarker abundance. Practical considerations for longitudinal CSF biobanking, including access device placement and collection, are also reviewed. Key takeaways and recommendations for CSF glioma biomarker discovery and validation are provided as a "hitchhiker's guide" based on our collective experience, along with resources for investigators aiming to develop CSF biobanking at their institutions.
Keywords: biomarker; cerebrospinal fluid; glioma; monitoring; neuro-oncology.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society for Neuro-Oncology.
Conflict of interest statement
The authors have no competing interests to declare that are directly related to the work in this review.
Figures



References
-
- Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

