A field resource for the glioma cerebrospinal fluid proteome: Impacts of resection and location on biomarker discovery
- PMID: 39786485
- PMCID: PMC12083222
- DOI: 10.1093/neuonc/noae277
A field resource for the glioma cerebrospinal fluid proteome: Impacts of resection and location on biomarker discovery
Abstract
Background: While serial sampling of glioma tissue is rarely performed prior to recurrence, cerebrospinal fluid (CSF) is an underutilized longitudinal source of candidate glioma biomarkers for understanding therapeutic impacts. However, the impact of key variables to consider in longitudinal CSF samples for monitoring biomarker discovery, including anatomical location and post-surgical changes, remains unknown.
Methods: Aptamer-based proteomics was performed on 147 CSF samples from 74 patients; 71 of whom had grade 2-4 astrocytomas or grade 2-3 oligodendrogliomas. This included pre- versus post-resection intracranial CSF samples obtained at early (1-16 days; n = 20 patients) or delayed (86-153 days; n = 11 patients) time points for patients with glioma. Paired lumbar versus intracranial glioma CSF samples were also obtained (n = 14 patients).
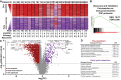
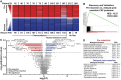
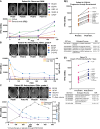
Results: Significant differences were identified in the CSF proteome between lumbar, subarachnoid, and ventricular CSF in patients with gliomas. Importantly, we found that resection had a significant, evolving longitudinal impact on the CSF proteome, with distinct sets of proteins present at different time points since resection. Our analysis of serial intracranial CSF samples suggests the early potential for disease monitoring and evaluation of pharmacodynamic impact of targeted therapies, such as bevacizumab and immunotherapies.
Conclusions: The intracranial glioma CSF proteome serves as a rich and dynamic reservoir of potential biomarkers that can be used to evaluate the effects of resection and other therapies over time. All data within this study, including detailed individual clinical annotations, are shared as a resource for the neuro-oncology community to collectively address these unanswered questions and further understand glioma biology through CSF proteomics.
Keywords: biomarker | cerebrospinal fluid | glioma | monitoring | proteomics.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society for Neuro-Oncology.
Conflict of interest statement
F.M. is a co-founder of and has equity in Harbinger Health, has equity in Zephyr AI, and consults for Harbinger Health and Zephyr AI. She serves on the board of directors of Exscientia Plc. She declares that none of these relationships are directly or indirectly related to the content of this manuscript.
Figures



References
-
- Weller M, Wick W, Aldape K, et al. Glioma. Nat Rev Dis Primers. 2015;1(1):15017. - PubMed
MeSH terms
Substances
Grants and funding
- Ivy Foundation
- Brains Together for the Cure
- R37 CA276851/CA/NCI NIH HHS/United States
- Humor to fight the Tumor
- Mayo Clinic Center for Individualized Medicine
- T32 GM145408/GM/NIGMS NIH HHS/United States
- Merck Pharmaceuticals
- U19 CA264362/CA/NCI NIH HHS/United States
- R61 NS122096/NS/NINDS NIH HHS/United States
- American Brain Tumor Association,
- UL1 TR002377/TR/NCATS NIH HHS/United States
- R37CA276851/the NIH National Cancer Institute
- UL1TR002377/CCaTS
- T32GM145408/National Institute of Health
- U19 CA2643632/the NIH National Cancer Institute
- Lucius & Terrie McKelvey
LinkOut - more resources
Full Text Sources
Medical

