Animal and vegetal materials of mouse oocytes segregate at first zygotic cleavage: a simple mechanism that makes the two-cell blastomeres differ reciprocally from the start
- PMID: 39786543
- PMCID: PMC11741683
- DOI: 10.1093/molehr/gaae045
Animal and vegetal materials of mouse oocytes segregate at first zygotic cleavage: a simple mechanism that makes the two-cell blastomeres differ reciprocally from the start
Abstract
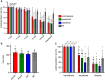
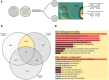
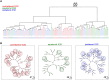
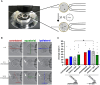
Recent advances in embryology have shown that the sister blastomeres of two-cell mouse and human embryos differ reciprocally in potency. An open question is whether the blastomeres became different as opposed to originating as different. Here we wanted to test two relevant but conflicting models: one proposing that each blastomere contains both animal and vegetal materials in balanced proportions because the plane of first cleavage runs close to the animal-vegetal axis of the fertilized oocyte (meridional cleavage); and the other model proposing that each blastomere contains variable proportions of animal and vegetal materials because the plane of the first cleavage can vary - up to an equatorial orientation - depending on the topology of fertilization. Therefore, we imposed the fertilization site in three distinct regions of mouse oocytes (animal pole, vegetal pole, equator) via ICSI. After the first zygotic cleavage, the sister blastomeres were dissociated and subjected to single-cell transcriptome analysis, keeping track of the original pair associations. Non-supervised hierarchical clustering revealed that the frequency of correct pair matches varied with the fertilization site (vegetal pole > animal pole > equator), thereby, challenging the first model of balanced partitioning. However, the inter-blastomere differences had similar signatures of gene ontology across the three groups, thereby, also challenging the competing model of variable partitioning. These conflicting observations could be reconciled if animal and vegetal materials were partitioned at the first cleavage: an event considered improbable and possibly deleterious in mammals. We tested this occurrence by keeping the fertilized oocytes immobilized from the time of ICSI until the first cleavage. Image analysis revealed that cleavage took place preferentially along the short (i.e. equatorial) diameter of the oocyte, thereby partitioning the animal and vegetal materials into the two-cell blastomeres. Our results point to a simple mechanism by which the two sister blastomeres start out as different, rather than becoming different.
Keywords: ICSI; animal model; blastomere; embryo development; fertilization; gene expression; oocyte.
© The Author(s) 2024. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Conflict of interest statement
M.B. is the current Editor-in-Chief of MHR (but had no involvement in any aspect of this article’s review process). The other authors declare no competing interests.
Figures



Similar articles
-
Retrospective analysis: reproducibility of interblastomere differences of mRNA expression in 2-cell stage mouse embryos is remarkably poor due to combinatorial mechanisms of blastomere diversification.Mol Hum Reprod. 2018 Jul 1;24(7):388-400. doi: 10.1093/molehr/gay021. Mol Hum Reprod. 2018. PMID: 29746690
-
The Principal Forces of Oocyte Polarity Are Evolutionary Conserved but May Not Affect the Contribution of the First Two Blastomeres to the Blastocyst Development in Mammals.PLoS One. 2016 Mar 31;11(3):e0148382. doi: 10.1371/journal.pone.0148382. eCollection 2016. PLoS One. 2016. PMID: 27030988 Free PMC article.
-
Focused time-lapse analysis reveals novel aspects of human fertilization and suggests new parameters of embryo viability.Hum Reprod. 2018 Jan 1;33(1):23-31. doi: 10.1093/humrep/dex344. Hum Reprod. 2018. PMID: 29149327
-
A systematic analysis of the suitability of preimplantation genetic diagnosis for mitochondrial diseases in a heteroplasmic mitochondrial mouse model.Hum Reprod. 2014 Apr;29(4):852-9. doi: 10.1093/humrep/deu016. Epub 2014 Feb 12. Hum Reprod. 2014. PMID: 24524987
-
From oocyte to 16-cell stage: cytoplasmic and cortical reorganizations that pattern the ascidian embryo.Dev Dyn. 2007 Jul;236(7):1716-31. doi: 10.1002/dvdy.21136. Dev Dyn. 2007. PMID: 17420986 Review.
Cited by
-
Production of live monkeys and their genetically matched embryonic stem cells from single embryos.Nat Commun. 2025 Jul 1;16(1):5529. doi: 10.1038/s41467-025-60694-5. Nat Commun. 2025. PMID: 40593544 Free PMC article.
References
-
- Antczak M, Van Blerkom J. Oocyte influences on early development: the regulatory proteins leptin and STAT3 are polarized in mouse and human oocytes and differentially distributed within the cells of the preimplantation stage embryo. Mol Hum Reprod 1997;3:1067–1086. - PubMed
-
- Antczak M, Van Blerkom J. Temporal and spatial aspects of fragmentation in early human embryos: possible effects on developmental competence and association with the differential elimination of regulatory proteins from polarized domains. Hum Reprod 1999;14:429–447. - PubMed
-
- Armant DR, Kaplan HA, Lennarz WJ. Fibronectin and laminin promote in vitro attachment and outgrowth of mouse blastocysts. Dev Biol 1986;116:519–523. - PubMed
-
- Athayde Wirka K, Chen AA, Conaghan J, Ivani K, Gvakharia M, Behr B, Suraj V, Tan L, Shen S. Atypical embryo phenotypes identified by time-lapse microscopy: high prevalence and association with embryo development. Fertil Steril 2014;101:1637–1648 e1631-1635. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

