The Dutch Early-Stage Melanoma (D-ESMEL) study: a discovery set and validation cohort to predict the absolute risk of distant metastases in stage I/II cutaneous melanoma
- PMID: 39786688
- PMCID: PMC11799080
- DOI: 10.1007/s10654-024-01188-4
The Dutch Early-Stage Melanoma (D-ESMEL) study: a discovery set and validation cohort to predict the absolute risk of distant metastases in stage I/II cutaneous melanoma
Abstract
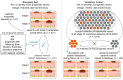
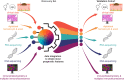
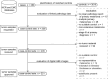
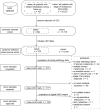
Early-stage cutaneous melanoma patients generally have a favorable prognosis, yet a significant proportion of metastatic melanoma cases arise from this group, highlighting the need for improved risk stratification using novel prognostic biomarkers. The Dutch Early-Stage Melanoma (D-ESMEL) study introduces a robust, population-based methodology to develop an absolute risk prediction model for stage I/II melanoma, incorporating clinical, imaging, and multi-omics data to identify patients at increased risk for distant metastases. Utilizing the Netherlands Cancer Registry and Dutch Nationwide Pathology Databank, we collected primary tumor samples from early-stage melanoma patients, with and without distant metastases during follow-up. Our study design includes a discovery set of metastatic cases and matched controls to identify novel prognostic factors, followed by a validation cohort using a nested case-control design to validate these factors and to build a risk prediction model. Tissue sections underwent Hematoxylin & Eosin (H&E) staining, RNA sequencing (RNAseq), DNA sequencing (DNAseq), immunohistochemistry (IHC), and multiplex immunofluorescence (MxIF).The discovery set included 442 primary melanoma samples (221 case-control sets), with 46% stage I and 54% stage II melanomas. The median time to distant metastasis was 3.4 years, while controls had a median follow-up time of 9.8 years. The validation cohort included 154 cases and 154 controls from a random population-based selection of 5,815 patients. Our approach enabled the collection of a large number of early-stage melanoma samples from population-based databases with extensive follow-up and a sufficient number of metastatic events. This methodology in prognostic cancer research holds the potential to impact clinical decision-making through absolute risk prediction.
Keywords: Biomarkers; Melanoma; Multi-omics; Population-based; Prognostic; Risk prediction.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interests: All authors declare no competing interests. Ethics approval: Ethical approval was obtained from the scientific committees of Erasmus MC (MEC-2018–1738), the Dutch Nationwide Pathology Databank, and the Netherlands Cancer Registry. Consent to participate: The use of leftover diagnostic tissue samples for scientific research is based on the ‘no objection’ principle, as outlined in the Code of Conduct for Health Research by the Committee on Regulation of Health Research.
Figures
References
-
- Garbe C, Keim U, Amaral T, et al. Prognosis of patients with primary melanoma stage I and II according to American joint committee on cancer version 8 validated in two independent cohorts: implications for adjuvant treatment. J Clin Oncol. 2022;40(32):3741–9. 10.1200/JCO.22.00202. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

