Clinical Decision Support and Cardiometabolic Medication Adherence: A Randomized Clinical Trial
- PMID: 39786775
- PMCID: PMC11718557
- DOI: 10.1001/jamanetworkopen.2024.53745
Clinical Decision Support and Cardiometabolic Medication Adherence: A Randomized Clinical Trial
Abstract
Importance: Medication adherence is important for managing blood pressure (BP), low-density lipoprotein cholesterol (LDL-C), and hemoglobin A1c (HbA1c). Interventions to improve medication adherence are needed.
Objective: To examine the effectiveness of an intervention using algorithmic identification of low medication adherence, clinical decision support to physicians, and pharmacist outreach to patients to improve cardiometabolic medication adherence and BP, LDL-C, and HbA1c control.
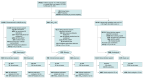
Design, setting, and participants: A 2-arm, patient-randomized, parallel group clinical trial was conducted. Twenty-six primary care clinics using effective decision support to encourage timely adjustments of cardiometabolic medications were included. On the date of an index visit, participants were (1) aged 18 to 75 years, (2) receiving a statin or not at the goal level for HbA1c or BP, and (3) had proportion of days covered less than 80% for 1 or more BP or noninsulin glucose-lowering medications or a statin. The study was conducted from August 19, 2020, to September 30, 2023. Data analysis was performed from October 1, 2023, to August 30, 2024.
Intervention: Electronic health record-linked clinical decision support identified and encouraged discussion of medication adherence issues. For patients in the intervention cohort continuing to meet eligibility criteria 6 months after an index visit, pharmacist telephone outreach was attempted.
Main outcomes and measures: The main outcomes of the trial were (1) adherence to selected classes of cardiometabolic medications, (2) control of HbA1c, BP, or LDL-C levels at 12 months after the index visit, and (3) costs of care.
Results: Among 5421 participants (2990 [55%] male; mean [SD] age, 57 [11] years) 12 months after the index date, intervention patients had better adherence to BP medications (adjusted odds ratio [AOR], 1.29; 95% CI, 1.06-1.56), but no better adherence to statins (AOR, 1.18; 95% CI, 0.99-1.41) or noninsulin diabetes medications (AOR, 1.03; 95% CI, 0.82-1.30) compared with patients receiving usual care. The intervention did not improve mean HbA1c (-0.2%; 95% CI, -0.4 to 0.1), systolic BP (1.4 mm Hg; 95% CI, -0.8 to 3.5 mm Hg), or LDL-C (-1.8 mg/dL; 95% CI, -6.5 to 2.8 mg/dL). Compared with usual care, intervention patients eligible for pharmacist outreach had improved HbA1c (-0.4%; 95% CI, -0.8% to -0.1%) compared with those not eligible for outreach (-0.0; 95% CI, -0.3% to 0.3%). Health care use costs did not differ significantly between study arms.
Conclusions and relevance: This cost-neutral intervention increased adherence to BP medications, but not to statins or glucose-lowering medications, with no overall improvement in BP, LDL-C, or HbA1c control. Modifications of this intervention strategy are needed to improve cardiometabolic risk factor control.
Trial registration: ClinicalTrials.gov Identifier: NCT03748420.
Conflict of interest statement
Figures


References
-
- World Health Organization . WHO. Adherence to long-term therapies: evidence for action. Accessed January 24, 2024. https://www.paho.org/en/documents/who-adherence-long-term-therapies-evid...
-
- Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43(6):521-530. doi:10.1097/01.mlr.0000163641.86870.af - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

