A high-throughput platform for single-molecule tracking identifies drug interaction and cellular mechanisms
- PMID: 39786807
- PMCID: PMC11717362
- DOI: 10.7554/eLife.93183
A high-throughput platform for single-molecule tracking identifies drug interaction and cellular mechanisms
Abstract
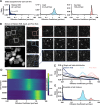
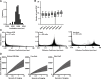
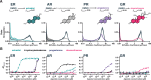
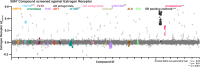
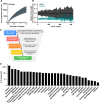
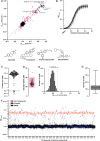
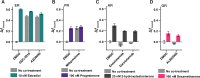
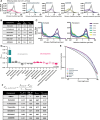
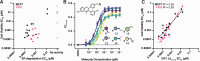
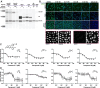
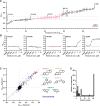
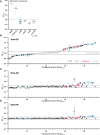
The regulation of cell physiology depends largely upon interactions of functionally distinct proteins and cellular components. These interactions may be transient or long-lived, but often affect protein motion. Measurement of protein dynamics within a cellular environment, particularly while perturbing protein function with small molecules, may enable dissection of key interactions and facilitate drug discovery; however, current approaches are limited by throughput with respect to data acquisition and analysis. As a result, studies using super-resolution imaging are typically drawing conclusions from tens of cells and a few experimental conditions tested. We addressed these limitations by developing a high-throughput single-molecule tracking (htSMT) platform for pharmacologic dissection of protein dynamics in living cells at an unprecedented scale (capable of imaging >106 cells/day and screening >104 compounds). We applied htSMT to measure the cellular dynamics of fluorescently tagged estrogen receptor (ER) and screened a diverse library to identify small molecules that perturbed ER function in real time. With this one experimental modality, we determined the potency, pathway selectivity, target engagement, and mechanism of action for identified hits. Kinetic htSMT experiments were capable of distinguishing between on-target and on-pathway modulators of ER signaling. Integrated pathway analysis recapitulated the network of known ER interaction partners and suggested potentially novel, kinase-mediated regulatory mechanisms. The sensitivity of htSMT revealed a new correlation between ER dynamics and the ability of ER antagonists to suppress cancer cell growth. Therefore, measuring protein motion at scale is a powerful method to investigate dynamic interactions among proteins and may facilitate the identification and characterization of novel therapeutics.
Keywords: cell biology; drug discovery; estrogen receptor; high-throughput imaging; human; live-cell imaging; physics of living systems; protein motion; single-molecule imaging.
© 2023, McSwiggen et al.
Conflict of interest statement
DM, HL, RT, SA, LA, RB, MB, HC, XD, KF, RG, EG, AH, AH, JH, SJ, RK, AK, RK, JL, KL, BM, PM, LM, SP, AS, CS, YT, DA, HB Employee of Eikon Therapeutics Inc
Figures





Update of
- doi: 10.1101/2023.01.05.522916
- doi: 10.7554/eLife.93183.1
- doi: 10.7554/eLife.93183.2
References
-
- Adams J, Palombella VJ, Sausville EA, Johnson J, Destree A, Lazarus DD, Maas J, Pien CS, Prakash S, Elliott PJ. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Research. 1999;59:2615–2622. - PubMed
-
- Barentine AES, Lin Y, Courvan EM, Kidd P, Liu M, Balduf L, Phan T, Rivera-Molina F, Grace MR, Marin Z, Lessard M, Rios Chen J, Wang S, Neugebauer KM, Bewersdorf J, Baddeley D. An integrated platform for high-throughput nanoscopy. Nature Biotechnology. 2023;41:1549–1556. doi: 10.1038/s41587-023-01702-1. - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

