Recruitment, follow-up and survival in an 11-country cohort study of patients at the end of life and their relatives
- PMID: 39787143
- PMCID: PMC11717288
- DOI: 10.1371/journal.pone.0317002
Recruitment, follow-up and survival in an 11-country cohort study of patients at the end of life and their relatives
Abstract
Background: Large, international cohort studies generate high-level evidence, but are resource intense. In end-of-life care such studies are scarce. Hence, planning for future studies in terms of data on screening, recruitment, retention and survival remains a challenge.
Objectives: The aim was to describe recruitment, follow-up and survival in a multinational study of patients' and relatives' expectations, concerns and preferences at the end of life.
Methods: In this 11-country cohort study with six months follow-up patients, >18 years old, were included on the basis of an adapted "surprise question" to assess patients´ end of life status. Patients were required to be aware of their limited life expectancy. We collected patient questionnaires (baseline and 1 month), and searched medical records for the date of death. One relative per patient was invited to participate.
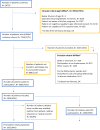
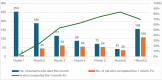
Results: 26735 patients were screened for inclusion, 3065 (11%) were found eligible and were invited to participate, 1509 chose to participate, i.e. 6% of those initially screened. A total of 699 patients (49%) participated in the 1-month follow-up, with proportions varying according to survival time, from 20% if the patient died at month 2, to 75% if the patient died at month 6. Survival time was not associated with patient gender or age, but with diagnosis, country of residence and healthcare setting.
Conclusion: Approximately 20 times the desired cohort size had to be screened for eligibility. Prognostication was difficult, we noted a wide distribution of survival after inclusion. Patients' ability to complete follow-up questionnaires declined well before death.
Copyright: © 2025 Schelin et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Trust N. Patient experience: do patients feel involved in decisions about their care? 2023. [Available from: https://www.nuffieldtrust.org.uk/resource/do-patients-feel-involved-in-d....
-
- Janssen DJ, Spruit MA, Schols JM, Wouters EF. Dynamic preferences for site of death among patients with advanced chronic obstructive pulmonary disease, chronic heart failure, or chronic renal failure. Journal of pain and symptom management. 2013;46(6):826–36. doi: 10.1016/j.jpainsymman.2013.01.007 - DOI - PubMed
-
- van Wijmen MPS, Pasman HRW, Twisk JWR, Widdershoven GAM, Onwuteaka-Philipsen BD. Stability of end-of-life preferences in relation to health status and life-events: A cohort study with a 6-year follow-up among holders of an advance directive. PLoS One. 2018;13(12):e0209315. doi: 10.1371/journal.pone.0209315 - DOI - PMC - PubMed
-
- Yildiz B aS, Bakan M et al.. Live well, die well–an international cohort study on experiences, concerns and preferences of patients in the last phase of life: the research protocol of the iLIVE study. BMJ Open. 2022.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical