Knockout of the ING5 epigenetic regulator confirms roles in stem cell maintenance and tumor suppression in vivo
- PMID: 39787145
- PMCID: PMC11717183
- DOI: 10.1371/journal.pone.0313255
Knockout of the ING5 epigenetic regulator confirms roles in stem cell maintenance and tumor suppression in vivo
Abstract
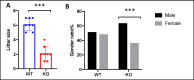
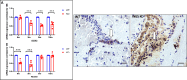
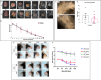
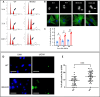
INhibitor of Growth (ING1-5) proteins are epigenetic readers that target histone acetyltransferase (HAT) or histone deacetylase (HDAC) complexes to the H3K4Me3 mark of active transcription. ING5 targets Moz/Morf and HBO1 HAT complexes that alter acetylation of H3 and H4 core histones, affecting gene expression. Previous experiments in vitro indicated that ING5 functions to maintain stem cell character in normal and in cancer stem cells. Here we find that CRISPR/Cas9 ING5 knockout (KO) mice are sub-fertile but show no decrease in lifespan or ability to heal wounds despite indications of depleted stem cell pools in several tissues. ING5 KO mouse embryo fibroblasts accumulate in G2 of the cell cycle, have high levels of abnormal nuclei and show high basal levels of the γH2AX indicator of DNA damage. KO animals also develop severe dermatitis at a 5-fold higher rate that wild-type littermates. Consistent with ING5 serving a tumor suppressive role, ING5 KO mice developed germinal centre diffuse large B-cell lymphomas at a rate 6-fold higher than control mice at 18 months of age. These data suggest that ING5 functions in vivo to maintain stem cell character in multiple organs, that reduction of stem cell populations is not limiting for murine lifespan and that like a subset of other ING family members, ING5 functions as a tumor suppressor in hematopoietic cells in vivo.
Copyright: © 2025 Al Shueili et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
All authors declare no competing interests for this study.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

