Azine Dearomatization in Natural Product Total Synthesis
- PMID: 39787324
- PMCID: PMC12080236
- DOI: 10.1002/chem.202402413
Azine Dearomatization in Natural Product Total Synthesis
Abstract
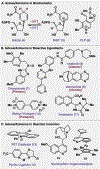
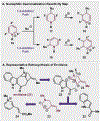
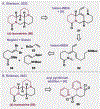
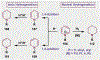
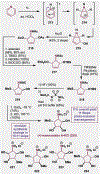
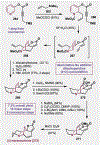
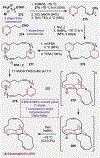
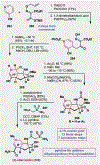
Since antiquity, alkaloid natural products have served as medicinal ingredients that still contribute as an inspiration for the development of novel therapeutics. For the synthetic chemist, much of the importance of natural products lies in their acting as a forcing-function for the invention of new synthetic strategies and tactics for molecular assembly. With this rich history in mind, it remains an important goal for chemists to build nitrogenous structures with greater efficiency, abiding by economies of synthesis. Nitrogenous aromatic feedstocks have been an intriguing starting point for the functionalization and construction of alkaloids for several decades, but recent advances in reaction design have opened new doors for leveraging their abundance in concise synthesis. Herein, advances in this area of synthetic ingenuity will be summarized with the aim of instructing chemists towards considering dearomatization as a strategic avenue for both target-oriented and diversity-oriented synthetic campaigns. Overall, syntheses are evaluated, compared, and contrasted to give a systematic overview of this continued area of research.
Keywords: Alkaloids; Dearomatization; Heterocycles; Pyridines; Total synthesis.
© 2025 Wiley-VCH GmbH.
Conflict of interest statement
Conflict of Interests
The authors declare no conflict of interest.
Figures

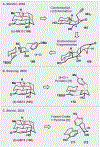

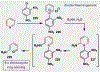
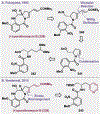
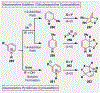
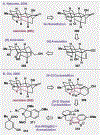
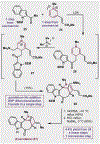
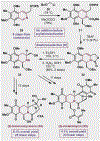
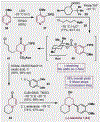
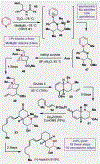

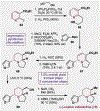
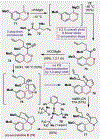

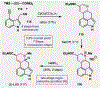
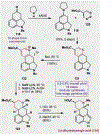
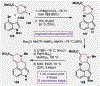

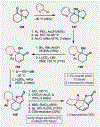
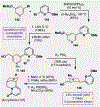
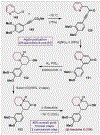
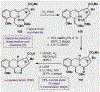

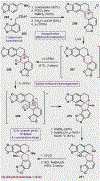



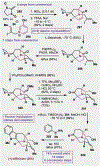

References
-
- Giseke AL, Arch. Pharm 1827, 20, 97–111.
-
- Ladenburg GB, Ber. Dtsch. Chem. Ges 1886, 19, 439–441.
-
- Eliot AC, Kirsch JF, Annu. Rev. Biochem 2004, 73, 383–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

