Safety, Pharmacokinetics, and Clinical Efficacy of ADS051, a Neutrophil Modulator, in Ulcerative Colitis: Results of a Randomized Phase 1b Trial
- PMID: 39787364
- PMCID: PMC12208385
- DOI: 10.14309/ajg.0000000000003269
Safety, Pharmacokinetics, and Clinical Efficacy of ADS051, a Neutrophil Modulator, in Ulcerative Colitis: Results of a Randomized Phase 1b Trial
Abstract
Introduction: Ulcerative colitis (UC) is characterized by colonic inflammation, with neutrophils playing a key role in UC activity, prognosis, and response to therapies. Current UC therapeutics can have significant side effects and limited efficacy. ADS051 is a novel, oral, gut-restricted small molecule that modulates neutrophil migration and activation without in vitro suppression of T-cell activation. The primary objective of this Phase 1b multidose trial was to evaluate the safety of ADS051. Secondary objectives were clinical activity and pharmacokinetics assessment.
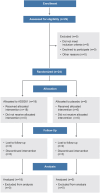
Methods: This trial enrolled 24 patients with moderate-to-severe UC in 3 sequential ascending dose cohorts with 3:1 randomization to ADS051 200 mg, 800 mg, or 3,200 mg, or placebo, administered orally once daily for 28 days. Safety, tolerability, and pharmacokinetics were assessed weekly, with clinical activity end points of clinical remission, endoscopic improvement, and histologic remission evaluated at Day 28.
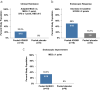
Results: ADS051 was well tolerated without severe or serious adverse events. High fecal concentrations were achieved with low systemic exposure, with <1% of the daily dose of ADS051 excreted in urine. On Day 28 of the trial, clinical remission was achieved in 22.2% of the pooled ADS051 group vs 0% of the pooled placebo group. Endoscopic response was achieved in 50.0% of ADS051-dosed vs 16.7% of placebo, and endoscopic improvement was achieved in 33.3% of ADS051-dosed vs 0% of placebo.
Discussion: Phase 1b data in patients with UC indicate a favorable safety profile for ADS051 with encouraging signals of clinical activity, supporting the advancement to a Phase 2 trial.
Trial registration: ClinicalTrials.gov NCT05084261.
Keywords: ADS051; gut-restricted; neutrophils; ulcerative colitis.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The American College of Gastroenterology.
Conflict of interest statement
Figures



References
-
- Danese S, Allez M, van Bodegraven AA, et al. Unmet medical needs in ulcerative colitis: An expert group consensus. Dig Dis 2019;37(4):266–83. - PubMed
-
- Zhou GX, Liu ZJ. Potential roles of neutrophils in regulating intestinal mucosal inflammation of inflammatory bowel disease. J Dig Dis 2017;18(9):495–503. - PubMed
-
- Ordás I, Eckmann L, Talamini M, et al. Ulcerative colitis. Lancet 2012;380(9853):1606–19. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

