Kinetic Oscillation Stimulation for the Preventive Treatment of Chronic Migraine: A Randomized, Double-Blind, Sham-Controlled Trial
- PMID: 39787477
- PMCID: PMC11720095
- DOI: 10.1212/WNL.0000000000210220
Kinetic Oscillation Stimulation for the Preventive Treatment of Chronic Migraine: A Randomized, Double-Blind, Sham-Controlled Trial
Abstract
Background and objectives: The Chordate System administers kinetic oscillation stimulation (K.O.S) into the nasal cavity thereby potentially modulating the activity of trigemino-autonomic reflex. Modulation of this reflex has been proposed as a potential therapeutic target in migraine. The aim of this clinical trial was to evaluate the efficacy of K.O.S for the preventive treatment of chronic migraine (CM).
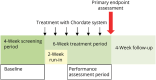
Methods: In this randomized, double-blind, sham-controlled, multicenter clinical trial, patients with CM were treated with K.O.S once per week over a period of 6 weeks. The primary performance endpoint was the mean change in monthly headache days with moderate to severe intensity (MHDs) from the 28-day pretreatment baseline period to the performance assessment period (days 14-42 of treatment). Mean change from baseline in monthly migraine days (MMDs), proportion of participants with 30% and 50% or greater reduction in moderate to severe headache days compared with baseline, and change in the use of abortive medications from baseline were also assessed during the performance assessment period. Headache-related disability and quality-of-life measures were evaluated up to 70-day posttreatment.
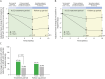
Results: The primary endpoint showed a significantly larger reduction of MHD across the performance assessment period with active treatment (-3.5 days, n = 67) compared with sham (-1.2 days, n = 65) (p = 0.0132). Compared with sham, active treatment consistently also led to significant reduction of MHD during the follow-up period (-2.7 [-4.3; -1.0, p = 0.0014]) as well as of mean MMDs during the assessment (-2.4 [-4.1; -0.7, p = 0.0048]) and follow-up (-2.9 [-4.5; -1.2, p = 0.0008]) periods. 61.8% of participants reported treatment-emergent adverse events (TEAEs) with similar incidences among treatment groups (63.2% [active], 60.3% [sham]), with nasopharyngitis (8.3%), dizziness (6.3%), and epistaxis (6.3%) being the most common TEAEs. Treatment-related serious adverse events were not observed.
Discussion: The Chordate System provides significant benefits to patients with CM by reducing the number of MHDs. The nonpharmacologic nature of the treatment option positions K.O.S as a valuable addition to the current therapeutic portfolio for the management of CM.
Trial registration information: The trial was registered on ClinicalTrials.gov (NCT03400059) on January 17, 2018. The first patient was enrolled on March 22, 2018, and the last patient completed the study on October 1, 2022. The trial registration initially described the timing of the secondary endpoints incorrectly due to clerical error, and this was corrected to match the protocol and analysis plan once discovered.
Classification of evidence: This study provides Class I evidence that weekly intranasal K.O.S is associated with a reduced number of headache days per month in patients with CM.
Conflict of interest statement
J. Hoffmann is currently a full-time employee of H. Lundbeck A/S. The clinical trial was finalized, and the publication submitted prior to his employment at H. Lundbeck A/S, while he was employed at King's College London. Before being employed at H. Lundbeck A/S, he received honoraria for consulting activities and/or serving on advisory boards and/or for giving lectures/presentations from AbbVie, Allergan, Autonomic Technologies Inc., Cannovex BV, Chordate Medical AB, MD-Horizonte, Eli Lilly, Hormosan Pharma, Lundbeck, Novartis, Pfizer, Sanofi, and Teva. He holds stock options from Chordate Medical AB. He received personal fees for Medico-Legal work as well as from NEJM Journal Watch, Oxford University Press, Quintessence Publishing, Sage Publishing, and Springer Healthcare. He also received research grants from Bristol Myers Squibb, International Headache Society (IHS), National Institute of Health and Care Research (NIHR), Medical Research Council (MRC), and the Migraine Trust. He serves as an associate editor for
Figures


References
-
- Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743-800. doi:10.1016/S0140-6736(15)60692-4 - DOI - PMC - PubMed
-
- Diener H-C, Förderreuther S, Gaul C, et al. . Prevention of migraine with monoclonal antibodies against CGRP or the CGRP receptor: addition to the S1 guideline: therapy of migraine attacks and prevention of migraine. Recommendations of the Germany Society of Neurology and the German Migraine and Headache Society. Neurol Res Pract. 2020;2:11. doi:10.1186/s42466-020-00057-1 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
