Exquisite sensitivity of Polycystin-1 to H2O2 concentration in the endoplasmic reticulum
- PMID: 39787899
- PMCID: PMC11763840
- DOI: 10.1016/j.redox.2024.103486
Exquisite sensitivity of Polycystin-1 to H2O2 concentration in the endoplasmic reticulum
Abstract
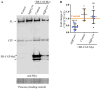
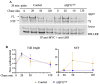
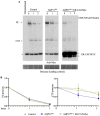
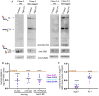
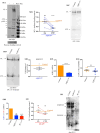
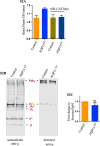
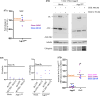
Aquaporin11 (AQP11) is an endoplasmic reticulum (ER) resident peroxiporin. It allows H2O2 transport from the lumen to the cytosol, guaranteeing redox homeostasis and signaling in and between the two organelles. Interestingly, Aqp11-/- mice develop a fatal, early onset polycystic kidney disease (PKD) similar to Autosomal Dominant PKD, a condition frequently associated with mutations of polycystin-1 (PC-1) in human patients. Here we investigated the molecular mechanisms of AQP11-associated PKD. Using different cell models, we show that transient downregulation of AQP11 selectively prevents the biogenesis of overexpressed PC-1. Expression of catalase in the ER lumen rescues the phenotype, demonstrating a direct role of (H2O2)ER in controlling the complex maturation of PC-1. Analysis of endogenous Pc-1 revealed an additional regulatory role at the pre-translational level. Taken together, our results show that AQP11 controls the complex biogenesis of PC-1 at multiple levels governing H2O2 intra and inter-organellar fluxes, with important implications in the pathogenesis and onset of PKD.
Keywords: Aquaporin 11; H(2)O(2); Polycystic kidney disease; Polycystin-1; Protein transport; Redox homeostasis.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest I declare that to the best of my knowledge the authors of this manuscript have no financial interests.
Figures








References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

