Determinants of maternal and infant omega-3 status at 3 months postpartum: findings from the APrON longitudinal cohort study
- PMID: 39788297
- PMCID: PMC11923373
- DOI: 10.1016/j.ajcnut.2025.01.002
Determinants of maternal and infant omega-3 status at 3 months postpartum: findings from the APrON longitudinal cohort study
Abstract
Background: Omega-3 long-chain-polyunsaturated fatty acids (LCPUFAs) are important dietary components for maternal and infant health during pregnancy and lactation.
Objectives: This study investigated determinants of maternal and infant LCPUFAs status at 3 mo postpartum and the relationship between maternal serum, maternal milk, and infant LCPUFAs.
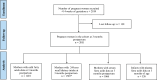
Methods: This cross-sectional study included mothers (n = 1481) and their offspring (n = 526) at 3 mo postpartum from the Alberta Pregnancy Outcomes and Nutrition (APrON) cohort. Maternal dietary intake (24-h recall), blood samples from mothers and infants, and maternal milk were collected. Fatty acid composition (relative % of total fatty acids) was determined by gas-liquid chromatography. Linear regression analyses explored associations between diet, sociodemographic factors, and fatty acid status.
Results: In a multivariable-adjusted analysis, maternal total dietary intake (supplement + food) was positively associated with the percentage of DHA (standardized ß [Sβ] = 0.158; ß = 0.394; 95% [confidence interval] CI: 0.192, 0.558; P < 0.001) in maternal serum phospholipids. Similar associations were found for DHA and eicosapentaenoic acid in maternal milk and plasma phospholipids of infants. Prepregnancy body mass index (BMI) was negatively associated with DHA (Sß = -0.073; ß = -0.003; 95% CI: -0.006, -0.001; P = 0.008) and positively associated with total saturated fatty acids (Sß = 0.086; ß = 0.111; 95% CI: 0.042, 0.180; P = 0.002) in maternal milk. Infants receiving formula combination with maternal milk had lower percentage of DHA (Sß = -0.177; ß = -0.390; 95% CI: -0.604, -0.175; P < 0.001) and arachidonic acid (Sß = -0.106; ß = -0.595; 95% CI: -1.122, -0.067; P = 0.027) in their plasma phospholipids compared with those who fed exclusively maternal milk.
Conclusions: Maternal total dietary intake and prepregnancy BMI are independently associated with their serum fatty acid status during lactation, whereas maternal diet, milk fatty acid composition, and lactation status are important determinants of infant n-3 LCPUFAs fatty acid status. Future research should investigate the impact of these differences in fatty acid status on infant health outcomes.
Keywords: dietary intake; docosahexaenoic acid; fatty acids; human milk; lactation.
Crown Copyright © 2025. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors report no conflicts of interest.
Figures

References
-
- U.S. Department of Agriculture . 9th Edition ed. 2020. Dietary Guidelines for Americans, 2020-2025. In: of USDoAaUSD, Services HaH.
-
- Gynecologists ACoOa Nutrition During Pregnancy [Internet] 2021. https://www.acog.org/womens-health/faqs/nutrition-during-pregnancy?utm_s...
-
- Health Canada Mercury in Fish - Questions and Answers [Internet] 2019. https://www.canada.ca/en/health-canada/services/food-nutrition/food-safe...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

