High-Throughput Determination of Exchange Rates of Unmodified and PTM-Containing Peptides Using HX-MS
- PMID: 39788320
- PMCID: PMC11875167
- DOI: 10.1016/j.mcpro.2025.100904
High-Throughput Determination of Exchange Rates of Unmodified and PTM-Containing Peptides Using HX-MS
Abstract
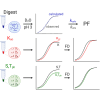
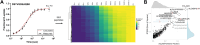
Despite the widespread use of MS for hydrogen/deuterium exchange measurements, no systematic, large-scale study has been conducted to compare the observed exchange rates in protein-derived, unstructured peptides measured by MS to the predicted exchange rates calculated from NMR-derived values and how neighboring residues and post-translational modifications influence those exchange rates. In this study, we sought to test the accuracy of predicted values by performing hydrogen exchange measurements on whole cell digests to generate an unbiased dataset of 563 unique peptides derived from naturally occurring protein sequences. A remarkable 97% of observed exchange rates of peptides are within two-fold of predicted values. Using fully deuterated controls, we found that for approximately 50% of the peptides, the amino acid sequence and, consequently, the intrinsic exchange rate, are the primary contributors to back exchange. A meta-analysis of the remaining physicochemical properties of peptides revealed multiple features that contribute either positively or negatively to back exchange discrepancies. Employing our workflow for comparable measurements on synthetic peptide mixtures containing post-translational modifications, and their unmodified counterparts, we show that lysine acetylation has a strong effect on the observed exchange rate, whereas serine/threonine phosphorylation does not. Our automated workflow enables high-throughput determination of exchange rates in complex biological peptide mixtures with diverse properties.
Keywords: acetylation; cell digest; exchange rates; hydrogen exchange; mass spectrometry; phosphorylation.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



References
-
- Connelly G.P., Bai Y., Jeng M.-F., Englander S.W. Isotope effects in peptide group hydrogen exchange. Proteins. 1993;17:87–92. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

