Inhibition of AXL ameliorates pulmonary fibrosis via attenuation of M2 macrophage polarisation
- PMID: 39788632
- PMCID: PMC12138030
- DOI: 10.1183/13993003.00615-2024
Inhibition of AXL ameliorates pulmonary fibrosis via attenuation of M2 macrophage polarisation
Abstract
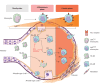
Rationale: Although a relationship between the growth arrest-specific 6 (GAS6)/anexelekto (AXL) pathway and pulmonary fibrosis has been suggested, the precise mechanisms and clinical implications of the AXL pathway in idiopathic pulmonary fibrosis are still unclear.
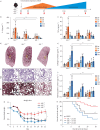
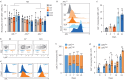
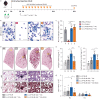
Methods: Constitutive and conditional AXL-knockout mice were generated and injected with bleomycin to induce pulmonary fibrosis. The expression of AXL and macrophage subtypes in bleomycin-injected mice and patients with idiopathic pulmonary fibrosis was analysed using flow cytometry. The therapeutic effects of the AXL inhibitors were examined.
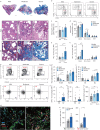
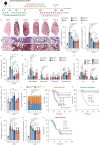
Results: AXL-deficient mice were resistant to bleomycin-induced pulmonary fibrosis and had a lower degree of M2-like macrophage differentiation than wild-type mice. Interestingly, AXL expression in monocytes was enhanced according to the progression of bleomycin-induced pulmonary fibrosis, and these results were especially prominent in lymphocyte antigen 6C (Ly6C)high monocytes. Gene silencing or inhibitor treatment with AXL inhibited the differentiation of M2-like macrophages during bone marrow-derived macrophage differentiation. These results were confirmed through experiments using AXLfl/flLysMCre+ mice and systems with depletion and reconstitution of macrophages. In line with these results, patients with severe idiopathic pulmonary fibrosis had high AXL expression in monocytes, high GAS6 levels and an enhanced population of M2-like macrophages compared to those with mild idiopathic pulmonary fibrosis. Lastly, treatment with AXL inhibitors ameliorated bleomycin-induced pulmonary fibrosis and improved survival rates.
Conclusions: The AXL pathway in classical monocytes contributes to pulmonary fibrosis progression through the induction of M2-like macrophage differentiation. Therefore, targeting AXL may be a promising therapeutic option for pulmonary fibrosis.
Copyright ©The authors 2025.
Conflict of interest statement
Conflict of interest: Y. Lee and Y. Yang are employees of Qurient, Seongnam, South Korea, which developed the QS0262 compound reported in this study. The remaining authors declare no potential conflicts of interest.
Figures


Comment in
-
Targeting the AXL pathway: a promising strategy for pulmonary fibrosis.Eur Respir J. 2025 Jun 5;65(6):2500114. doi: 10.1183/13993003.00114-2025. Print 2025 Jun. Eur Respir J. 2025. PMID: 40473304 No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
