Elevated CO2 alters soybean physiology and defense responses, and has disparate effects on susceptibility to diverse microbial pathogens
- PMID: 39788902
- PMCID: PMC12095978
- DOI: 10.1111/nph.20364
Elevated CO2 alters soybean physiology and defense responses, and has disparate effects on susceptibility to diverse microbial pathogens
Abstract
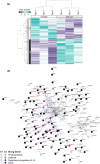
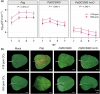
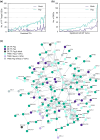
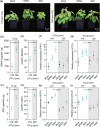
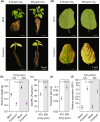
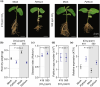
Increasing atmospheric CO2 levels have a variety of effects that can influence plant responses to microbial pathogens. However, these responses are varied, and it is challenging to predict how elevated CO2 (eCO2) will affect a particular plant-pathogen interaction. We investigated how eCO2 may influence disease development and responses to diverse pathogens in the major oilseed crop, soybean. Soybean plants grown in ambient CO2 (aCO2, 419 parts per million (ppm)) or in eCO2 (550 ppm) were challenged with bacterial, viral, fungal, and oomycete pathogens. Disease severity, pathogen growth, gene expression, and molecular plant defense responses were quantified. In eCO2, plants were less susceptible to Pseudomonas syringae pv. glycinea (Psg) but more susceptible to bean pod mottle virus, soybean mosaic virus, and Fusarium virguliforme. Susceptibility to Pythium sylvaticum was unchanged, although a greater loss in biomass occurred in eCO2. Reduced susceptibility to Psg was associated with enhanced defense responses. Increased susceptibility to the viruses was associated with reduced expression of antiviral defenses. This work provides a foundation for understanding how future eCO2 levels may impact molecular responses to pathogen challenges in soybean and demonstrates that microbes infecting both shoots and roots are of potential concern in future climatic conditions.
Keywords: Fusarium virguliforme; Glycine max; Pseudomonas syringae; Pythium sylvaticum; bean pod mottle virus; carbon dioxide; plant immunity; soybean mosaic virus.
© 2025 The Author(s). New Phytologist © 2025 New Phytologist Foundation.
Conflict of interest statement
None declared.
Figures



References
-
- Aguilar E, Allende L, Del Toro FJ, Chung B‐N, Canto T, Tenllado F. 2015. Effects of elevated CO2 and temperature on pathogenicity determinants and virulence of potato virus X/potyvirus‐associated synergism. Molecular Plant–Microbe Interactions 28: 1364–1373. - PubMed
-
- Ahammed GJ, Li X. 2022. Elevated carbon dioxide‐induced regulation of ethylene in plants. Environmental and Experimental Botany 202: 105025.
-
- Ainsworth EA, Long SP. 2021. 30 years of free‐air carbon dioxide enrichment (FACE): what have we learned about future crop productivity and its potential for adaptation? Global Change Biology 27: 27–49. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

