Catalytic acceptorless complete dehydrogenation of cycloalkanes
- PMID: 39788935
- PMCID: PMC11718209
- DOI: 10.1038/s41467-024-55460-y
Catalytic acceptorless complete dehydrogenation of cycloalkanes
Abstract
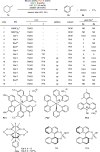
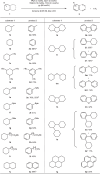
The advancement of an effective hydrogen liberation technology from liquid organic hydrogen carriers, particularly cycloalkanes such as cyclohexane and methylcyclohexane, holds significance in realizing a hydrogen-centric society. However, the attainment of homogeneous catalytic acceptorless dehydrogenation characterized by elevated selectivity for thorough aromatization under mild conditions remains unrealized. In this study, a catalyst system, facilitated by a double hydrogen atom transfer processes, has been devised for the catalytic acceptorless dehydrogenation of inert cycloalkanes at ambient temperature under visible light irradiation. Through the synergistic utilization of tetrabutylammonium chloride and thiophosphoric acid hydrogen atom transfer catalysts, successful catalytic acceptorless dehydrogenation with comprehensive aromatization has been accomplished with potential liquid organic hydrogen carrier candidates and showcased high functional group tolerance.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
-
- Gupta, M., Chrystel, H., Flesher, R. J., Kaska, W. C., & Jensen, C. M. A highly active alkane dehydrogenation catalyst: stabilization of dihydrido rhodium and iridium complexes by a P–C–P pincer ligand. Chem. Commun. 2083–2084 (1996).
-
- Xu, W.-W., et al Thermochemical alkane dehydrogenation catalyzed in solution without the use of a hydrogen acceptor. Chem. Commun. 2273–2274 (1997).
Grants and funding
LinkOut - more resources
Full Text Sources

