Microfluidic platforms for monitoring cardiomyocyte electromechanical activity
- PMID: 39788940
- PMCID: PMC11718118
- DOI: 10.1038/s41378-024-00751-z
Microfluidic platforms for monitoring cardiomyocyte electromechanical activity
Abstract
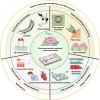
Cardiovascular diseases account for ~40% of global deaths annually. This situation has revealed the urgent need for the investigation and development of corresponding drugs for pathogenesis due to the complexity of research methods and detection techniques. An in vitro cardiomyocyte model is commonly used for cardiac drug screening and disease modeling since it can respond to microphysiological environmental variations through mechanoelectric feedback. Microfluidic platforms are capable of accurate fluid control and integration with analysis and detection techniques. Therefore, various microfluidic platforms (i.e., heart-on-a-chip) have been applied for the reconstruction of the physiological environment and detection of signals from cardiomyocytes. They have demonstrated advantages in mimicking the cardiovascular structure and function in vitro and in monitoring electromechanical signals. This review presents a summary of the methods and technologies used to monitor the contractility and electrophysiological signals of cardiomyocytes within microfluidic platforms. Then, applications in common cardiac drug screening and cardiovascular disease modeling are presented, followed by design strategies for enhancing physiology studies. Finally, we discuss prospects in the tissue engineering and sensing techniques of microfluidic platforms.
© 2025. The Author(s).
Conflict of interest statement
Conflict of interest: The authors declare no competing interests.
Figures




References
-
- World Health Organization. Invisible Numbers: The True Extent of Noncommunicable Diseases and What to Do About Them (World Health Organization, 2022).
-
- World Health Organization. World Health Statistics 2023: Monitoring Health for the SDGs, Sustainable Development Goals (World Health Organization, 2023).
-
- Congressional Budget Office. Research and Development in the Pharmaceutical Industry.https://www.cbo.gov/publication/57126 (2021).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
