Adhesive and injectable hydrogel microspheres for NRF2-mediated periodontal bone regeneration
- PMID: 39788942
- PMCID: PMC11717957
- DOI: 10.1038/s41368-024-00340-w
Adhesive and injectable hydrogel microspheres for NRF2-mediated periodontal bone regeneration
Abstract
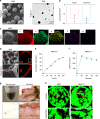
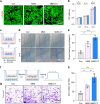
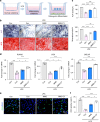
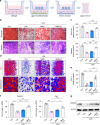
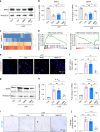
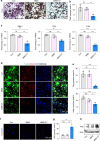
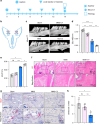
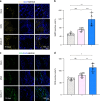
Regenerating periodontal bone defect surrounding periodontal tissue is crucial for orthodontic or dental implant treatment. The declined osteogenic ability of periodontal ligament stem cells (PDLSCs) induced by inflammation stimulus contributes to reduced capacity to regenerate periodontal bone, which brings about a huge challenge for treating periodontitis. Here, inspired by the adhesive property of mussels, we have created adhesive and mineralized hydrogel microspheres loaded with traditional compound cordycepin (MMS-CY). MMS-CY could adhere to the surface of alveolar bone, then promote the migration capacity of PDLSCs and thus recruit them to inflammatory periodontal tissues. Furthermore, MMS-CY rescued the impaired osteogenesis and ligament-forming capacity of PDLSCs, which were suppressed by the inflammation stimulus. Moreover, MMS-CY also displayed the excellent inhibitory effect on the osteoclastic activity. Mechanistically, MMS-CY inhibited the premature senescence induced by the inflammation stimulus through the nuclear factor erythroid 2-related factor (NRF2) pathway and reducing the DNA injury. Utilizing in vivo rat periodontitis model, MMS-CY was demonstrated to enhance the periodontal bone regeneration by improving osteogenesis and inhibiting the osteoclastic activity. Altogether, our study indicated that the multi-pronged approach is promising to promote the periodontal bone regeneration in periodontitis condition by reducing the inflammation-induced stem cell senescence and maintaining bone homeostasis.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

