Influenza A Virus H7 nanobody recognizes a conserved immunodominant epitope on hemagglutinin head and confers heterosubtypic protection
- PMID: 39788944
- PMCID: PMC11718266
- DOI: 10.1038/s41467-024-55193-y
Influenza A Virus H7 nanobody recognizes a conserved immunodominant epitope on hemagglutinin head and confers heterosubtypic protection
Abstract
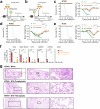
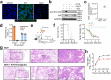
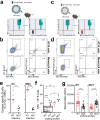
Influenza remains a persistent global health challenge, largely due to the virus' continuous antigenic drift and occasional shift, which impede the development of a universal vaccine. To address this, the identification of broadly neutralizing antibodies and their epitopes is crucial. Nanobodies, with their unique characteristics and binding capacity, offer a promising avenue to identify such epitopes. Here, we isolate and purify a hemagglutinin (HA)-specific nanobody that recognizes an H7 subtype of influenza A virus. The nanobody, named E10, exhibits broad-spectrum binding, cross-group neutralization and in vivo protection across various influenza A subtypes. Through phage display and in vitro characterization, we demonstrate that E10 specifically targets an epitope on HA head which is part of the conserved lateral patch and is highly immunodominant upon H7 infection. Importantly, immunization with a peptide including the E10 epitope elicits cross-reactive antibodies and mediates partial protection from lethal viral challenge. Our data highlights the potential of E10 and its associated epitope as a candidate for future influenza prevention strategies.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Barry, J. M. The Great Influenza: The Story of the Deadliest Pandemic in History (Penguin UK, 2020).
-
- Organization W. H. Global Influenza Strategy 2019-2030 (World Health Organization, 2019).
MeSH terms
Substances
Grants and funding
- 850638/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- 2021-01165/Vetenskapsrådet (Swedish Research Council)
- 2021-01164/Vetenskapsrådet (Swedish Research Council)
- 2021.0033/Knut och Alice Wallenbergs Stiftelse (Knut and Alice Wallenberg Foundation)
- U23A20243/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

