The NE/AAT/CBG axis regulates adipose tissue glucocorticoid exposure
- PMID: 39788946
- PMCID: PMC11718191
- DOI: 10.1038/s41467-024-55693-x
The NE/AAT/CBG axis regulates adipose tissue glucocorticoid exposure
Abstract
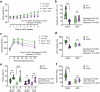
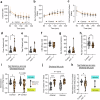
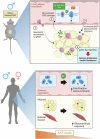
Corticosteroid binding globulin (CBG; SERPINA6) binds >85% of circulating glucocorticoids but its influence on their metabolic actions is unproven. Targeted proteolytic cleavage of CBG by neutrophil elastase (NE; ELANE) significantly reduces CBG binding affinity, potentially increasing 'free' glucocorticoid levels at sites of inflammation. NE is inhibited by alpha-1-antitrypsin (AAT; SERPINA1). Using complementary approaches in mice and humans to manipulate NE or AAT, we show high-fat diet (HFD) increases the NE:AAT ratio specifically in murine visceral adipose tissue, an effect only observed in males. Notably, HFD-fed male mice lacking NE have reduced glucocorticoid levels and action specifically in visceral adipose tissue, with improved glucose tolerance and insulin sensitivity, independent of systemic changes in free glucocorticoids. The protective effect of NE deficiency is lost when the adrenals are removed. Moreover, human asymptomatic heterozygous carriers of deleterious mutations in SERPINA1 resulting in lower AAT levels have increased adipose tissue glucocorticoid levels and action. However, in contrast to mice, humans present with systemic increases in free circulating glucocorticoid levels, an effect independent of HPA axis activation. These findings show that NE and AAT regulate local tissue glucocorticoid bioavailability in vivo, providing crucial evidence of a mechanism linking inflammation and metabolism.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

