Molecular logic for cellular specializations that initiate the auditory parallel processing pathways
- PMID: 39788966
- PMCID: PMC11717940
- DOI: 10.1038/s41467-024-55257-z
Molecular logic for cellular specializations that initiate the auditory parallel processing pathways
Abstract
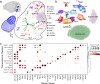
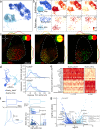
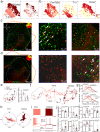
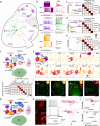
The cochlear nuclear complex (CN), the starting point for all central auditory processing, encompasses a suite of neuronal cell types highly specialized for neural coding of acoustic signals. However, the molecular logic governing these specializations remains unknown. By combining single-nucleus RNA sequencing and Patch-seq analysis, we reveal a set of transcriptionally distinct cell populations encompassing all previously observed types and discover multiple hitherto unknown subtypes with anatomical and physiological identity. The resulting comprehensive cell-type taxonomy reconciles anatomical position, morphological, physiological, and molecular criteria, enabling the determination of the molecular basis of the specialized cellular phenotypes in the CN. In particular, CN cell-type identity is encoded in a transcriptional architecture that orchestrates functionally congruent expression across a small set of gene families to customize projection patterns, input-output synaptic communication, and biophysical features required for encoding distinct aspects of acoustic signals. This high-resolution account of cellular heterogeneity from the molecular to the circuit level reveals the molecular logic driving cellular specializations, thus enabling the genetic dissection of auditory processing and hearing disorders with a high specificity.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


Update of
-
Molecular logic for cellular specializations that initiate the auditory parallel processing pathways.bioRxiv [Preprint]. 2024 Oct 6:2023.05.15.539065. doi: 10.1101/2023.05.15.539065. bioRxiv. 2024. Update in: Nat Commun. 2025 Jan 9;16(1):489. doi: 10.1038/s41467-024-55257-z. PMID: 37293040 Free PMC article. Updated. Preprint.
Similar articles
-
Molecular logic for cellular specializations that initiate the auditory parallel processing pathways.bioRxiv [Preprint]. 2024 Oct 6:2023.05.15.539065. doi: 10.1101/2023.05.15.539065. bioRxiv. 2024. Update in: Nat Commun. 2025 Jan 9;16(1):489. doi: 10.1038/s41467-024-55257-z. PMID: 37293040 Free PMC article. Updated. Preprint.
-
The projection from auditory cortex to cochlear nucleus in guinea pigs: an in vivo anatomical and in vitro electrophysiological study.Exp Brain Res. 2003 Dec;153(4):467-76. doi: 10.1007/s00221-003-1606-2. Epub 2003 Sep 18. Exp Brain Res. 2003. PMID: 14504855
-
Modulatory influences on time-coding neurons in the ventral cochlear nucleus.Hear Res. 2019 Dec;384:107824. doi: 10.1016/j.heares.2019.107824. Epub 2019 Oct 17. Hear Res. 2019. PMID: 31670183 Review.
-
Linear coding of complex sound spectra by discharge rate in neurons of the medial nucleus of the trapezoid body (MNTB) and its inputs.Front Neural Circuits. 2014 Dec 16;8:144. doi: 10.3389/fncir.2014.00144. eCollection 2014. Front Neural Circuits. 2014. PMID: 25565971 Free PMC article.
-
The ion channels and synapses responsible for the physiological diversity of mammalian lower brainstem auditory neurons.Hear Res. 2019 May;376:33-46. doi: 10.1016/j.heares.2018.12.011. Epub 2018 Dec 26. Hear Res. 2019. PMID: 30606624 Review.
Cited by
-
Cellular and synaptic specializations for sub-millisecond precision in the mammalian auditory brainstem.Front Cell Neurosci. 2025 May 19;19:1568506. doi: 10.3389/fncel.2025.1568506. eCollection 2025. Front Cell Neurosci. 2025. PMID: 40458470 Free PMC article. Review.
-
Molecular Cascades That Build and Connect Auditory Neurons from Hair Cells to the Auditory Cortex.J Exp Neurol. 2025;6(3):111-120. J Exp Neurol. 2025. PMID: 40823538 Free PMC article.
-
Fusiform Cells in the Dorsal Cochlear Nucleus Change Intrinsic Electrophysiological Properties and Morphologically Remodel Their Basal Dendrites with Age.bioRxiv [Preprint]. 2025 Jul 21:2025.07.16.665173. doi: 10.1101/2025.07.16.665173. bioRxiv. 2025. PMID: 40777380 Free PMC article. Preprint.
-
Architecture, dynamics and biogenesis of GluA3 AMPA glutamate receptors.Nature. 2025 Jul 1. doi: 10.1038/s41586-025-09325-z. Online ahead of print. Nature. 2025. PMID: 40592473
References
-
- Oertel, D., McGinley, M. & and Cao, X.-J. in Encylopedia of Neuroscience (Springer, 2009).
-
- Grothe, B., Pecka, M. & McAlpine, D. Mechanisms of sound localization in mammals. Physiol. Rev.90, 983–1012 (2010). - PubMed
-
- Chi, T., Ru, P. & Shamma, S. A. Multiresolution spectrotemporal analysis of complex sounds. J. Acoust. Soc. Am.118, 887–906 (2005). - PubMed
-
- Rhode, W. S. & Greenberg, S. in The Mammalian Auditory Pathway: Neurophysiology (eds. Arthur N. Popper & Richard R. Fay) 94–152 (Springer, 1992).
-
- Palmer, A. R. Physiology of the cochlear nerve and cochlear nucleus. Br. Med. Bull.43, 838–855 (1987). - PubMed
MeSH terms
Grants and funding
- R35NS116798/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R01NS110767/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- P30 EY002520/EY/NEI NIH HHS/United States
- R01DC004450/U.S. Department of Health & Human Services | NIH | National Institute on Deafness and Other Communication Disorders (NIDCD)
- R01 NS110767/NS/NINDS NIH HHS/United States
- P50 HD103555/HD/NICHD NIH HHS/United States
- R01DC017797/U.S. Department of Health & Human Services | NIH | National Institute on Deafness and Other Communication Disorders (NIDCD)
- R35 NS116798/NS/NINDS NIH HHS/United States
- S10 OD016167/OD/NIH HHS/United States
- R01 DC017797/DC/NIDCD NIH HHS/United States
- R01 MH120404/MH/NIMH NIH HHS/United States
- R01 DC004450/DC/NIDCD NIH HHS/United States
- R01MH122169; R01MH120404/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)
- R01 MH122169/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

