Neoadjuvant or concurrent atezolizumab with chemoradiation for locally advanced cervical cancer: a randomized phase I trial
- PMID: 39788967
- PMCID: PMC11718273
- DOI: 10.1038/s41467-024-55200-2
Neoadjuvant or concurrent atezolizumab with chemoradiation for locally advanced cervical cancer: a randomized phase I trial
Abstract
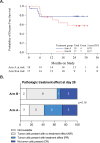
Combined immune checkpoint blockade (ICB) and chemoradiation (CRT) is approved in patients with locally advanced cervical cancer (LACC) but optimal sequencing of CRT and ICB is unknown. NRG-GY017 (NCT03738228) was a randomized phase I trial of atezolizumab (anti-PD-L1) neoadjuvant and concurrent with CRT (Arm A) vs. concurrent with CRT (Arm B) in patients with high-risk node-positive LACC. The primary endpoint was the fraction of expanded tumor-associated T-cell receptor (TCR) clones in blood at day 21 as a surrogate measure of anti-tumor immune response. Secondary objectives were safety and feasibility, 2-year disease-free survival (DFS), and predictive value of PD-L1 expression. Forty patients were randomized, 36 received treatment, and 25 were evaluable for the primary endpoint. After cycle 1, there was peripheral expansion of higher proportion of tumor-associated TCR clones in Arm A than in Arm B (p = 0.0025) that remained higher at day 21, meeting the pre-specified endpoint on two-sample T-test (p = 0.052), but not on sensitivity analysis by Wilcoxon test (p = 0.13). At the median follow up of 25.8 months, 2-year DFS was 76% in Arm A and 56% in Arm B (p = 0.28). There were no new safety signals. In conclusion, neoadjuvant ICB prior to CRT was safe and was associated with immunologically and clinically favorable outcomes, warranting larger confirmatory studies.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Jyoti Mayadev reports personal fees from AstraZeneca, Merck, Primmune, Varian Medical Systems, The GOG Foundation, Inc; grant support from NCI, NRG Oncology, Moores Cancer Center UCSD, The GOG Foundation, Inc. Dmitriy Zamarin reports institutional grants from Merck, Genentech, AstraZeneca, Plexxikon, and Synthekine, and personal fees from AstraZeneca, Xencor, Memgen, Takeda, Synthekine, Immunos, Tessa Therapeutics, Miltenyi, and Calidi Biotherapeutics. DZ owns a patent on the use of oncolytic Newcastle Disease Virus for cancer therapy. Junzo Chino received personal consulting fees from Stryker. He also received a stipend for chapter writing (personal) from the GOG Foundation. He participated on a Data Safety Monitoring Board for KM Pharmaceutical Consulting LLC. He also served as an unpaid Board Member for the American Brachytherapy Society. Barbara Banbury is employed by and holds stock in Adaptive Biotechnologies. Ned Sherry is employed by and holds stock in Adaptive Biotechnologies. Sharad Ghamande received clinical trial payments to the institution from Merck, GSK/Tejaro, Jovance, Clovis, Oncology, Takedo, and Eisai. He received payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing, or educational events from GSK and Eisai. Loren Mell’s institution received grants or contracts from Merck and AstraZeneca. He also received consulting fees from Cel-Sci as well as payment for expert testimony from Sanofi. He participated on a Data Safety Monitoring Board for Pfizer. Cara Mathews’ institution received funding from the National Cancer Institute. She also received grants or contracts from Syros, Deciphera, Astellas Pharma, Seagen, Genmab, EMD Serono, Merck, Regeneron, Moderna, AstraZeneca, AvengeBio, Zentalis, GlaxoSmithKline, and Genentech outside the submitted work. David O’Malley received support from the NRG/NCI/GOG Foundation. He also received grants or contractd from AbbVie, Advaxis, Agenus, Inc., Alkermes, Aravive, Inc., Arcus Biosciences, Inc., AstraZeneca, BeiGene USA, Inc., Boston Biomedical, Bristol Myers Squibb, Clovis Oncology, Deciphera Pharma, Eisai, EMD Serono, Inc., Exelixis, Genentech Inc., Genmab, GlaxoSmithKline, GOG Foundation, Hoffmann-LaRoche Inc., ImmunoGen, Inc., Incyte Corporation, IOVANCE, Biotherapeutics, Karyopharm, Leap Therapeutics, Inc., Ludwig Institute for Ca, Merck & Co., Merck Sharp and Dohme Corp., Mersana Therapeutics, Inc., NCI, Novartis, Novocure, NRG Oncology, OncoC4, Inc., OncoQuest Inc., Pfizer Inc., Precision Therapeutics, Inc., Prelude Therapeutics, Regeneron Pharmaceuticals, Inc., RTOG, Rubius Therapeutics, Seattle Genetics (SeaGen), Sutro BioPharma, SWOG, TESARO, Verastem, Inc. Dr. O’Malley reports personal fees from Consulting and/or advisory board member from AbbVie, AdaptImmune, Agenus,Inc, Arquer Diagnostics, Arcus Biosciences, Inc., AstraZeneca, Atossa Therapeutics, Boston Biomedical, Cardiff Oncology, Celcuity, Clovis Oncology, Corcept Therapeutics, Duality Bio, Eisai, Elevar, Exelixis, Genentech Inc, Genelux, GlaxoSmithKline, GOG Foundation, Hoffmann-La Roche Inc, ImmunoGen, Inc, Imvax, InterVenn, INXMED, IOVANCE Biotherapeutics, Janssen, Jazz Pharmaceuticals, Laekna, Leap Therapeutics, Inc., Luzsana Biotechology, Merck & Co, Merck Sharp & Dohme Corp., Mersana Therapeutics,Inc, Myriad, Novartis, NovoCure, OncoC4, Inc., Onconova, Regeneron Pharmaceuticals, Inc, RepImmune, R Pharm, Roche Diagnostics, Seattle Genetics (SeaGen), Sorrento, Sutro Biopharma, Tarveda Therapeutics, Toray, Trillium, Umoja, Verastem, Inc, VBL Therapeutics, Vincerx Pharma, Xencor, and Zentalis. Alexander Olawaiye received an Honorarium for Advisory Board Meetings from AstraZeneca, GSK, and Merck. He also received a grant payment made to his institution from AstraZeneca. Elizabeth Hopp participated in the Immunogen Advisory Board on 6/1/23. Charles Leath contracted research with AstraZeneca and Merck. He also received a consulting/Scientific Advisory Board with Merck and Seattle Genetics. Larry Copeland received payment to himself, with institutional approval from the GOG Foundation President. He also received support for travel to GOG Semiannual meetings based on receipts. Robert Mannel received payment from his institution for NCI/NRG trial-based capitation. Roisin O’Cearbhaill received support from NCI/NIH (grant P30 CA008748). She also received grants or contracts paid to her institution from: Bayer/Celgene/Juno, Tesaro/GSK, Merck, Ludwig Cancer Institute, Abbvie/StemCentrx, Regeneron, TCR2 Therapeutics, Atara Biotherapeutics, Marker Therapeutics, Syndax Pharmaceuticals, Genmab/Seagen Therapeutics, Genentech, Alkermes, Kite Pharma, Acrivon and Gynecologic Oncology Foundation, Lyell Immunopharma, Bayer/Celgene/Juno, Tesaro/GSK, Merck, Ludwig Cancer Institute, Abbvie/StemCentrx, Regeneron, TCR2 Therapeutics, Atara Biotherapeutics, Marker Therapeutics, Syndax Pharmaceuticals, Genmab/Seagen Therapeutics, Genentech, Alkermes, Kite Pharma, Acrivon and Gynecologic Oncology Foundation, Lyell Immunopharma. She also received payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing, or educational events from GSK, Ciro/Onclive/PER/MJH/Aptitude Health, SITC, and Gynecologic Oncology Canada. She received support for attending meetings and/or travel from Hitech Health, Gathering Around Cancer, Ireland, GOG Foundation, and SGO. She participated on a Data Safety Monitoring Board or Advisory Board from AstraZeneca (DUO-0), GSK (Moonstone, Prma) and Acrivon for unpaid steering committee, Carina Biotech, Link Therapeutics, Tesaro/GSK, Regeneron Advisory, Seattle Genetics/SeaGen, Immunogen board, Bayer, R-Pharm, Fresenius Kabi, Miltenyi, 2Seventybio and Bayer for advisory boards. She had a leadership or fiduciary role as Vice-Chair for CPC, SGO Chair, CT Committee, and NRG Oncology. Carol Aghajanian received clinical trial funding to her institution (MSK) as follows: Abbvie – MSK, PI, GOG 3005, AstraZeneca – MSK PI, SOLO1/GOG 3004, National Coordinating Investigator & MSK PI, DO81RC00001; ENGOT – ov 46; AGO-OVAR 23; GOG−3025; Clovis – MSK PI, ARIEL 2 & 3; Genentech/Roche – MSK PI, GOG 3015 (IMagyn050). She also received consulting fees from Roche/Genentech – Advisory Board 8/21/20; Eisai/Merck – Advisory Board 9/12/20; AstraZeneca/Merck – Advisory Boards 9/30/20 & 10/14/20 and Repare Therapeutics – Advisory Board 10/15/20. She participated on an Advisory Board 6/30/21 for Blueprint Medicine. She served on GOG Foundation, Board of Directors (unpaid, occasional travel cost reimbursement to attending meetings); NRG Oncology Board of Directors (unpaid). Russell Schilder received consulting fees from Incyte, and Honoraria from Pfizer and participated on a Data Safety Monitoring Board or Advisory Board for Celsion. All other co-authors have no Competing Interests to declare.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- U10 CA180868/CA/NCI NIH HHS/United States
- R50 CA282102/CA/NCI NIH HHS/United States
- UG1 CA233330/CA/NCI NIH HHS/United States
- P50 CA098252/CA/NCI NIH HHS/United States
- NRG Oncology U10CA180822 (NRG Oncology Statistics and Data Management Center)/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- U10CA180868 (NRG Oncology Operations),/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- R01CA276087/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- U24 CA196067/CA/NCI NIH HHS/United States
- R50CA282102-01/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- R01 CA276087/CA/NCI NIH HHS/United States
- U10 CA180822/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- P30-CA008748/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)

