Finite element investigation for improving chest wall reconstruction process using ceramic and polymeric implants
- PMID: 39788988
- PMCID: PMC11718210
- DOI: 10.1038/s41598-024-79536-3
Finite element investigation for improving chest wall reconstruction process using ceramic and polymeric implants
Abstract
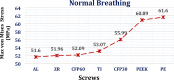
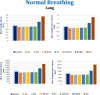
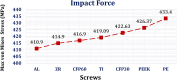
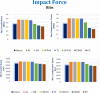
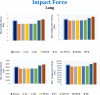
Car accidents, infections caused by bacteria or viruses, metastatic lesions, tumors, and malignancies are the most frequent causes of chest wall damage, leading to the removal of the affected area. After excision, artificial bone or synthetic materials are used in chest wall reconstruction to restore the skeletal structure of the chest. Chest implants have traditionally been made from metallic materials like titanium alloys due to their biocompatibility and durability. However, the drawbacks of these materials have prompted researchers to seek alternative materials for use in the reconstruction process. This research aims to explore alternatives to metallic implants in order to overcome their drawbacks and enhance the performance of chest wall reconstruction using the finite element method. In this research, customized implants for the ribs and cartilages are used to repair the defective portion of the chest wall. The implants are made from various materials, including stiff bioceramics (alumina and zirconia), soft polymers (polyether ether ketone (PEEK) and polyethylene (PE)), and polymeric composites (carbon fiber-reinforced PEEK 30 and 60% (CFP 30 and 60%)) as alternatives for titanium. They are tested under normal breathing and impact loading conditions. The null hypothesis suggests that stiff implants will provide optimal results. The results illustrate that when using alumina implants, under normal breathing, the maximum tensile and compressive stresses increased to 11.41 and 15.86 MPa on ribs, while decreasing to 0.32 and 0.324 MPa, and 0.96 and 0.56 Pa on cartilages and lung respectively, compared to titanium. Conversely, when using PE implants, the maximum tensile and compressive stresses decreased to 5.69 and 8.2 MPa on ribs and increased to 0.4 and 0.42 MPa, and 1.71 and 1.1 MPa on cartilages and lung respectively. Under impact force, compared to titanium, the maximum tensile and compressive stresses increased to 47.5 and 49.8 MPa on ribs, and decreased to 1.91 and 6.15 MPa, and 4.56 and 7.7 Pa on cartilages and lung respectively, when using alumina implants. On the other hand, the maximum tensile and compressive stresses decreased to 31 and 23 MPa on ribs and increased to 2.52 and 7.83 MPa, and 5.8 and 9.3 MPa on cartilages and lung respectively, when using PE implants. The highest tensile and compressive strains on ribs were 6,162 and 6,235 µε when using alumina implants under impact force. Additionally, the highest tensile and compressive strains on cartilages and lung were 11,192 and 20,918 µε and 5,836 and 9,335 µε, respectively, when using PE implants. For screws, the peak values of von Mises stress were 61.6 MPa and 433.4 MPa under normal breathing and impact force respectively, when using PE implants. In fatigue analysis, alumina, PEEK, and PE implants failed under impact force as the maximum equivalent alternating stresses exceeded their fatigue limits, resulting in safety factors of less than one. It was concluded that stiff bioceramic implants (alumina and zirconia) produced the lowest stresses and strains on the surrounding cartilages and underlying lung, and the highest stresses and strains on the surrounding ribs, unlike soft PEEK and PE implants. Additionally, CFP 30% and 60% implants distributed stresses on the ribs, cartilages, and lungs similarly to titanium implants. Furthermore, the tensile and compressive stresses and strains on the ribs, cartilages, and lungs did not exceed allowable limits for all used implants. Finally, Zirconia, CFP 30%, and CFP 60% implants can be used as substitutes for titanium in chest wall reconstruction to restore damaged portions of the ribs and cartilage. However, stiff alumina implants and soft PEEK & PE implants were not recommended for use as they were susceptible to fracture under impact force.
Keywords: Alumina; Carbon fiber-reinforced PEEK; Chest implants; Chest wall reconstruction; PE; PEEK; Zirconia.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The author declares no competing interests.
Figures

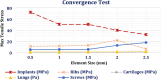












References
-
- Iaizzo, P. A. Handbook of Cardiac anatomy, physiology, and devices. J. Card. Surg.21, 327 (2006). - DOI
-
- Saxena, A. K. Chest Wall Deformities (Springer, 2017).
-
- 3 & Roy, S. Chest Wall Tumors: A Comprehensive Review Book (Blue Rose, 2024).
-
- Gai Gaissert, H. A. Disorders of the Chest Wall, an Issue of Thoracic Surgery Clinics, E-Book (Elsevier Health Sciences, 2017).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

