Y2O3NPs induce selective cytotoxicity, genomic instability, oxidative stress and ROS mediated mitochondrial apoptosis in human epidermoid skin A-431 Cancer cells
- PMID: 39789066
- PMCID: PMC11718274
- DOI: 10.1038/s41598-024-82376-w
Y2O3NPs induce selective cytotoxicity, genomic instability, oxidative stress and ROS mediated mitochondrial apoptosis in human epidermoid skin A-431 Cancer cells
Abstract
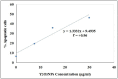
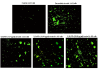
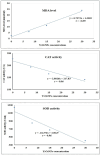
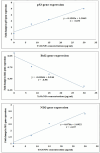
Yttrium oxide nanoparticles (Y2O3NPs) have emerged as a promising avenue for cancer therapy, primarily due to their distinctive properties that facilitate selective targeting of cancer cells. Despite their potential, the therapeutic effects of Y2O3NPs on human epidermoid skin cancer remain largely unexplored. This study was thus conducted to investigate the impact of Y2O3NPs on both human skin normal and cancer cells, with an emphasis on assessing their cytotoxicity, genotoxicity, and the mechanisms underlying these effects. Cell viability and apoptosis induction were assessed using the Sulforhodamine B and chromatin diffusion assay, respectively. Reactive oxygen species (ROS) level, mitochondrial membrane potential integrity, oxidative stress markers and expression level of apoptotic and mitochondrial genes were also estimated. Our findings highlight the selective and significant cytotoxicity of Y2O3NPs against human epidermoid A-431 cancer cells. Notably, exposure to five Y2O3NPs concentrations (0.1, 1, 10, 100 and 1000 µg/ml) resulted in a high concentration-dependent reduction in cell viability and a corresponding increase in cell death observed 72 h post-treatment specifically in A-431 cancer cells, while normal skin fibroblast (HSF) cells exhibited minimal toxicity. When A-431 cancer cells were treated with the half-maximal inhibitory concentration (IC50) of Y2O3NPs for 72 h, a significant increase in ROS generation was noted. This led to oxidative stress, along with severe damage to genomic DNA and mitochondrial membrane potential, triggering substantial apoptosis. Furthermore, a concurrent significant upregulation of apoptotic p53 and mitochondrial ND3 genes was observed, coupled with a notable decrease in the anti-apoptotic Bcl2 gene expression.Overall, Y2O3NPs demonstrate considerable promise as a therapeutic agent for skin epidermoid cancer due to their ability to selectively target and induce cytotoxic effects in A-431 cancer cells, all while causing minimal harm to normal HSF cells. This selective cytotoxicity appears to be associated with Y2O3NPs' ability to induce excessive ROS production and subsequent oxidative stress, leading to significant genomic DNA fragmentation, loss of mitochondrial permeability, and alterations in apoptotic and mitochondrial genes' expression, ultimately promoting apoptosis in A-431 cancer cells. These findings establish a foundation for further research into the utilization of Y2O3NPs in targeted cancer therapies and underscore the necessity for ongoing investigation into their safety and efficacy in clinical applications.
Keywords: Cytotoxicity, genotoxicity, apoptosis; Oxidative stress and mitochondria; Yttrium oxide nanoparticles.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
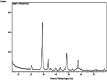
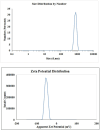

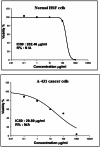


Similar articles
-
Yttrium oxide nanoparticles induce selective cytotoxicity, genomic instability and ROS mitochondrial P53 mediated apoptosis in human pancreatic cancer cells.Sci Rep. 2025 Jun 20;15(1):20144. doi: 10.1038/s41598-025-05088-9. Sci Rep. 2025. PMID: 40542009 Free PMC article.
-
Yttrium oxide nanoparticles ameliorates calcium hydroxide and calcium titanate nanoparticles induced genomic DNA and mitochondrial damage, ROS generation and inflammation.Sci Rep. 2024 Jun 6;14(1):13015. doi: 10.1038/s41598-024-62877-4. Sci Rep. 2024. PMID: 38844752 Free PMC article.
-
Calcium titanate nanoparticles-induced cytotoxicity, genotoxicity and oxidative stress in human non-small lung cancer cells.Sci Rep. 2025 Feb 21;15(1):6373. doi: 10.1038/s41598-025-89035-8. Sci Rep. 2025. PMID: 39984527 Free PMC article.
-
Investigating the Anticancer Potential of Biochanin A in KB Oral Cancer Cells Through the NFκB Pathway.Cell Biochem Funct. 2024 Sep;42(7):e4130. doi: 10.1002/cbf.4130. Cell Biochem Funct. 2024. PMID: 39364853 Review.
-
Anti-aging Strategies and Topical Delivery of Biopolymer-based Nanocarriers for Skin Cancer Treatment.Curr Aging Sci. 2024;17(1):31-48. doi: 10.2174/1874609816666230320122018. Curr Aging Sci. 2024. PMID: 36941817 Review.
Cited by
-
Erbium oxide nanoparticles induce potent cell death, genomic instability and ROS-mitochondrial dysfunction-mediated apoptosis in U937 lymphoma cells.Naunyn Schmiedebergs Arch Pharmacol. 2025 Aug;398(8):11027-11039. doi: 10.1007/s00210-025-03962-x. Epub 2025 Mar 12. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40072553 Free PMC article.
-
Potent cytotoxicity and induction of ROS-mediated genomic instability, mitochondrial dysfunction, and apoptosis by Y2O3 NPs in Hep-G2 hepatic cancer cells.Naunyn Schmiedebergs Arch Pharmacol. 2025 Apr 10. doi: 10.1007/s00210-025-04051-9. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40208319
References
-
- Lomas, A., Leonardi-Bee, J. & Bath-Hextall, F. Risk factors for non-melanoma skin cancer: a systematic review. Br. J. Dermatol.184 (4), 737–748 (2021).
-
- Hollebeek, C., Braham, R. & Franco, C. Role of UV radiation in the development of squamous cell carcinoma. Am. J. Dermatology. 32 (3), 274–280 (2020).
-
- Nair, S., Papadopoulos, K. & Thompson, P. Mohs micrographic surgery for high-risk squamous cell carcinoma. J. Dermatol. Surg.45 (5), 561–568 (2019).
-
- Gordon, K., Goh, C. & Duvic, M. Topical and systemic treatments for non-melanoma skin cancers. Dermatol. Ther., 35(4), e15395. (2022).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

