Primary staging with 2[18F]-FDG-PET/CT and -PET/MRI and radiotherapy response evaluation with MRI in uterine cervical cancer: an interim analysis of a prospective clinical trial
- PMID: 39789229
- PMCID: PMC11718017
- DOI: 10.1186/s41824-024-00236-2
Primary staging with 2[18F]-FDG-PET/CT and -PET/MRI and radiotherapy response evaluation with MRI in uterine cervical cancer: an interim analysis of a prospective clinical trial
Abstract
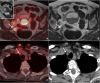
Background: In uterine cervical cancer (UCC), tumour staging is performed according to the 2018 International Federation of Gynecology and Obstetrics (FIGO) system, where imaging is incorporated, or the more generic Tumour Node Metastasis (TNM) classification. With the technical development in diagnostic imaging, continuous prospective evaluation of the different imaging methods contributing to stage determination is warranted. The aims of this interim study were to (1) evaluate the performance of radiological FIGO (rFIGO) and T staging (rT) with 2-fluorine-18-fluoro-deoxy-glucose (2[18F]-FDG)-positron emission tomography with computed tomography (PET/CT) and with magnetic resonance imaging (PET/MRI), compared to clinical FIGO (cFIGO) and T (cT) staging based on clinical examination and conventional imaging, in treatment-naïve UCC, and to (2) identify possible MRI biomarkers for early treatment response after radiotherapy.
Methods: Ten consecutive patients with newly diagnosed UCC from the prospective PRODIGYN (Prognostic and Diagnostic Added Value of Medical Imaging in Staging and Treatment Planning of Gynecological Cancer) study (ethical approval number 2022-04207-01; NCT05855941) were included. Study participants underwent 2[18F]FDG-PET/CT and -PET/MRI, and an additional MRI one week after radiotherapy. Agreement between rFIGO and cFIGO was analysed using Cohen's kappa. Differences in rFIGO between 2[18F]FDG-PET/CT and -PET/MRI were evaluated with Wilcoxon signed ranks test, and added value of rFIGO for metastasis assessment was demonstrated with descriptive statistics.
Results: In 2/10 patients, a higher stage was obtained with rFIGO compared to cFIGO, where presence of metastases led to upstaging. In 3/10, rFIGO was lower than cFIGO, and in 5/10 rFIGO and cFIGO were similar. Degree of agreement between rFIGO and cFIGO was poor, (κ = 0.091, p < 0.005) with 2[18F]FDG-PET/CT and (κ = - 0.010, p > 0.05) with FDG/PET/MRI). There was no significant difference between 2[18F]FDG-PET/CT and -PET/MRI for rFIGO (p = 0.18), or rT stage assessment (p = 0.32). MRI-derived tumour volume and apparent diffusion coefficient (ADC) were most affected on MRI one week after radiotherapy.
Conclusions: Our results indicate that there is an added value of rFIGO staging with 2[18F]FDG-PET/CT and -PET/MRI compared to clinical examination and conventional radiology, for metastasis assessment in treatment-naïve UCC. In early treatment response evaluation with MRI, ADC and tumour volume may be predictive parameters of interest in future prognostic analyses.
Trial registration: Clinical Trials, NCT05855941. Registered 02 May 2023, https://clinicaltrials.gov/study/NCT05855941?term=NCT05855941&rank=1 .
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The PRODIGYN study (Prognostic and Diagnostic Added Value of Medical Imaging in Staging and Treatment Planning of Gynecological Cancer) was approved by the Swedish Ethical Review Authority with ethical approval number 2022-04207-01; NCT05855941. Study participants were given oral and written information and had the opportunity to ask questions. Written informed consent was obtained from all study participants. Consent for publication: Written informed consent was obtained from all study participants. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Added value of radiological staging to clinical examination in different histopathological subtypes of uterine cervical cancer: A retrospective study.Eur J Obstet Gynecol Reprod Biol X. 2025 Mar 6;26:100376. doi: 10.1016/j.eurox.2025.100376. eCollection 2025 Jun. Eur J Obstet Gynecol Reprod Biol X. 2025. PMID: 40129448 Free PMC article.
-
Combined pre-treatment MRI and 18F-FDG PET/CT parameters as prognostic biomarkers in patients with cervical cancer.Eur J Radiol. 2014 Jul;83(7):1169-1176. doi: 10.1016/j.ejrad.2014.03.024. Epub 2014 Mar 30. Eur J Radiol. 2014. PMID: 24767630
-
Prospective Comparison of 18F-Choline Positron Emission Tomography/Computed Tomography (PET/CT) and 18F-Fluorodeoxyglucose (FDG) PET/CT in the Initial Workup of Multiple Myeloma: Study Protocol of a Prospective Imaging Trial.JMIR Res Protoc. 2020 Sep 10;9(9):e17850. doi: 10.2196/17850. JMIR Res Protoc. 2020. PMID: 32909953 Free PMC article.
-
18F-FDG PET/CT and whole-body MRI diagnostic performance in M staging for non-small cell lung cancer: a systematic review and meta-analysis.Eur Radiol. 2020 Jul;30(7):3641-3649. doi: 10.1007/s00330-020-06703-1. Epub 2020 Mar 3. Eur Radiol. 2020. PMID: 32125513
-
Diagnostic role of 18F-FDG PET/MRI in the TNM staging of breast cancer: a systematic review and meta-analysis.Ann Palliat Med. 2021 Apr;10(4):4328-4337. doi: 10.21037/apm-20-2555. Epub 2021 Apr 12. Ann Palliat Med. 2021. PMID: 33894709
References
-
- Beiderwellen K, Grueneisen J, Ruhlmann V, Buderath P, Aktas B, Heusch P et al (2015) [(18)F]FDG PET/MRI vs. PET/CT for whole-body staging in patients with recurrent malignancies of the female pelvis: initial results. Eur J Nucl Med Mol Imaging 42(1):56–65 - PubMed
-
- Cancercentrum (2024) Nationellt vårdprogram livmoderhals- och vaginalcancer [2024–01–25]. Available from: https://kunskapsbanken.cancercentrum.se/diagnoser/livmoderhals-och-vagin...
-
- Das S, Chandramohan A, Reddy JK, Mukhopadhyay S, Kumar RM, Isiah R et al (2015) Role of conventional and diffusion weighted MRI in predicting treatment response after low dose radiation and chemotherapy in locally advanced carcinoma cervix. Radiother Oncol 117(2):288–293 - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
