Human iPSC-derived microglial cells protect neurons from neurodegeneration in long-term cultured adhesion brain organoids
- PMID: 39789340
- PMCID: PMC11718079
- DOI: 10.1038/s42003-024-07401-0
Human iPSC-derived microglial cells protect neurons from neurodegeneration in long-term cultured adhesion brain organoids
Abstract
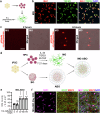
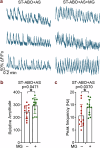
Brain organoid models have greatly facilitated our understanding of human brain development and disease. However, key brain cell types, such as microglia, are lacking in most brain organoid models. Because microglia have been shown to play important roles in brain development and pathologies, attempts have been made to add microglia to brain organoids through co-culture. However, only short-term microglia-organoid co-cultures can be established, and it remains challenging to have long-lasting survival of microglia in organoids to mimic long-term residency of microglia in the brain. In this study, we developed an adhesion brain organoid (ABO) platform that allows prolonged culture of brain organoids (greater than a year). Moreover, the long-term (LT)-ABO system contains abundant astrocytes and can support prolonged survival and ramification of microglia. Furthermore, we showed that microglia in the LT-ABO could protect neurons from neurodegeneration by increasing synaptic density and reducing p-Tau level and cell death in the LT-ABO. Therefore, the microglia-containing LT-ABO platform generated in this study provides a promising human cellular model for studying neuron-glia and glia-glia interactions in brain development and the pathogenesis of neurodegenerative diseases such as Alzheimer's disease.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

