Co-Expression of Tardive Dyskinesia and Drug-Induced Parkinsonism in Rats Chronically Treated With Haloperidol
- PMID: 39789385
- PMCID: PMC11717661
- DOI: 10.1002/npr2.12524
Co-Expression of Tardive Dyskinesia and Drug-Induced Parkinsonism in Rats Chronically Treated With Haloperidol
Abstract
Aim: We aimed to create a rat model of drug-induced parkinsonism and tardive dyskinesia by chronic administration of haloperidol and examine the expression of direct and indirect pathway markers in the striatum of the model rats.
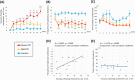
Methods: We treated 21 rats, 14 with haloperidol decanoate and 7 with placebo. The number of vacuous chewing movements per 2 min was counted, and haloperidol-treated rats were classified into two groups: mild and severe tardive dyskinesia. Other behavioral analyses were also conducted. After a 6-month treatment period, rat brains were removed, and protein expression was evaluated by Western blotting.
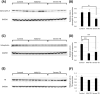
Results: All haloperidol-treated rats exhibited vacuous chewing movements. The frequency of exploratory behavior and rotarod test performance was lower in the mild and severe tardive dyskinesia groups. The number of vacuous chewing movements and frequency of exploratory behavior were positively correlated in haloperidol-treated rats. The expression of dynorphin, a direct pathway marker, decreased in the severe tardive dyskinesia group. The expression of enkephalin, an indirect pathway marker, decreased both in the mild and severe tardive dyskinesia groups. The expression of dopamine D1 and D2 receptors also decreased with haloperidol treatment.
Conclusion: Both direct and indirect pathways are involved in haloperidol-induced movement disorders.
Keywords: direct pathway; dopamine D1 receptor; dopamine D2 receptor; dynorphin; enkephalin; indirect pathway; vacuous chewing movement.
© 2025 The Author(s). Neuropsychopharmacology Reports published by John Wiley & Sons Australia, Ltd on behalf of The Japanese Society of Neuropsychopharmacology.
Conflict of interest statement
Haruo Nishijima received a research grant from Sumitomo Pharma Co. Ltd. The other authors declare that they have no competing financial interests or personal relationships that could have influenced the work reported in this study.
Figures

Similar articles
-
Pharmacological and neurochemical differences between acute and tardive vacuous chewing movements induced by haloperidol.Psychopharmacology (Berl). 1996 Oct;127(4):337-45. doi: 10.1007/s002130050095. Psychopharmacology (Berl). 1996. PMID: 8923569
-
Differential effects of antipsychotics on haloperidol-induced vacuous chewing movements and subcortical gene expression in the rat.Eur J Pharmacol. 2003 Sep 12;477(2):101-12. doi: 10.1016/j.ejphar.2003.08.018. Eur J Pharmacol. 2003. PMID: 14519413
-
Quantitative autoradiography of striatal dopamine D1, D2 and re-uptake sites in rats with vacuous chewing movements.Brain Res. 1994 May 23;646(2):217-22. doi: 10.1016/0006-8993(94)90081-7. Brain Res. 1994. PMID: 8069667
-
Tardive dyskinesia: pathophysiology and animal models.J Clin Psychiatry. 2000;61 Suppl 4:5-9. J Clin Psychiatry. 2000. PMID: 10739324 Review.
-
Relevance of animal models to human tardive dyskinesia.Behav Brain Funct. 2012 Mar 9;8:12. doi: 10.1186/1744-9081-8-12. Behav Brain Funct. 2012. PMID: 22404856 Free PMC article. Review.
References
-
- Tarsy D., “Neuroleptic‐Induced Extrapyramidal Reactions: Classification, Description, and Diagnosis,” Clinical Neuropharmacology 6, no. Suppl 1 (1983): S9–S26. - PubMed
-
- Carbon M., Hsieh C. H., Kane J. M., and Correll C. U., “Tardive Dyskinesia Prevalence in the Period of Second‐Generation Antipsychotic Use: A Meta‐Analysis,” Journal of Clinical Psychiatry 78, no. 3 (2017): e264–e278. - PubMed
-
- Gunne L. M. and Andren P. E., “An Animal Model for Coexisting Tardive Dyskinesia and Tardive Parkinsonism: A Glutamate Hypothesis for Tardive Dyskinesia,” Clinical Neuropharmacology 16, no. 1 (1993): 90–95. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

