Continued Treatment with Nintedanib in Patients with Progressive Pulmonary Fibrosis: Data from INBUILD-ON
- PMID: 39789408
- PMCID: PMC11717875
- DOI: 10.1007/s00408-024-00778-z
Continued Treatment with Nintedanib in Patients with Progressive Pulmonary Fibrosis: Data from INBUILD-ON
Abstract
Purpose: In the INBUILD trial in patients with progressive pulmonary fibrosis (PPF), nintedanib slowed the decline in forced vital capacity (FVC) versus placebo, with a safety profile characterised mainly by gastrointestinal events. INBUILD-ON, the open-label extension of INBUILD, assessed the safety of nintedanib during longer-term treatment. Data on FVC were collected.
Study design and methods: Adverse events and changes in FVC in INBUILD-ON were assessed descriptively in all patients and in two subgroups: patients who received nintedanib in INBUILD and continued nintedanib in INBUILD-ON ("continued nintedanib" group) (n = 212) and patients who received placebo in INBUILD and initiated nintedanib in INBUILD-ON ("initiated nintedanib" group) (n = 222). Changes in FVC were based on observed values.
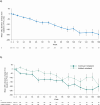
Results: Median exposure to nintedanib in INBUILD-ON was 22.0 months. Diarrhoea was the most frequent adverse event. Amongst patients who had diarrhoea, 90.0% experienced only events of mild or moderate severity. Adverse events led to discontinuation of nintedanib at a rate of 16.7 per 100 patient-years. Serious and fatal adverse events were reported at rates of 37.2 and 9.5 per 100 patient-years. Mean (SE) changes in FVC from baseline to week 48 were - 71.6 (16.1) mL [- 128.5 (25.5) mL in continued nintedanib group (n = 106), - 14.8 (18.2) mL in initiated nintedanib group (n = 106)].
Conclusion: The safety profile of nintedanib in INBUILD-ON was consistent with that in INBUILD. Change in FVC in INBUILD-ON was consistent with decline in FVC in the nintedanib group of INBUILD. These results support the use of nintedanib in the long-term treatment of PPF.
Clinical trial registration: ClinicalTrials.gov; NCT03820726; registered January 29, 2019.
Keywords: Clinical trial; Drug tolerance; Interstitial lung disease; Pulmonary function tests; Vital capacity.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: WAW reports grants paid to his institution from Boehringer Ingelheim, Galapagos, Roche, and Alentis. FB has served as an advisor or review panel member for Boehringer Ingelheim, Bristol Myers Squibb (BMS), Fujirebio, Galapagos, GlaxoSmithKline (GSK), Roche, and Takeda; received fees for speaking from Boehringer Ingelheim, Fujirebio, Galapagos, and Roche; and support for travel from Boehringer Ingelheim and Roche. NC reports consulting fees from Boehringer Ingelheim, Bridge Therapeutics, Liminal BioSciences, Redex, Transcript, and Vicore and fees for speaking from Boehringer Ingelheim. FV reports consulting fees from Boehringer Ingelheim and fees for speaking from Boehringer Ingelheim and Roche. DA-O reports grants from Boehringer Ingelheim, FibroGen, Galapagos, Galecto, Genentech/Roche, and Pliant. JWS reports grants from the National Research Foundation of Korea, the Korea National Institute of Health, and the Korea Environment Industry & Technology Institute; he reports fees for speaking from Boehringer Ingelheim, BMS, and Eisai; and has served as a consultant and steering committee member for Boehringer Ingelheim, Taiho, and Daewoong. CM is an employee of mainanalytics GmbH, Sulzbach (Taunus), Germany, which was contracted by Boehringer Ingelheim to assist with these analyses. MD and CC are employees of Boehringer Ingelheim. VC reports grants from Boehringer Ingelheim; fees for speaking from Boehringer Ingelheim, Roche, and Ferrer; consulting fees from Boehringer Ingelheim, Celgene/BMS, CSL Behring, Ferrer, Galapagos, GSK, Pliant, PureTech, Redx, Roche, Sanofi, and Shionogi; has served as an advisor or review panel member for Celgene/BMS, FibroGen, Galapagos, Galecto, Roche; and has received support for travel from Boehringer Ingelheim, Ferrer, and Roche. Ethical Approval: The INBUILD and INBUILD-ON trials were conducted in accordance with the principles of the Declaration of Helsinki and the Harmonised Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonisation. The study protocols were reviewed and approved by an Independent Ethics Committee and/or Institutional Review Board at each trial site. Consent to Participate: All patients provided written informed consent before study entry. Consent for Publication: Not applicable.
Figures

References
-
- Wijsenbeek M, Cottin V (2020) Spectrum of fibrotic lung diseases. N Engl J Med 383:958–968 - PubMed
-
- Flaherty KR, Wells AU, Cottin V et al (2019) Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med 381:1718–1727 - PubMed
-
- George PM, Spagnolo P, Kreuter M et al (2020) Progressive fibrosing interstitial lung disease: clinical uncertainties, consensus recommendations, and research priorities. Lancet Respir Med 8:925–934 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

