Secreted LGALS3BP facilitates distant metastasis of breast cancer
- PMID: 39789641
- PMCID: PMC11715970
- DOI: 10.1186/s13058-024-01958-8
Secreted LGALS3BP facilitates distant metastasis of breast cancer
Abstract
Background: Patients with estrogen receptor (ER)-positive breast cancer (BC) can be treated with endocrine therapy targeting ER, however, metastatic recurrence occurs in 25% of the patients who have initially been treated. Secreted proteins from tumors play important roles in cancer metastasis but previous methods for isolating secretory proteins had limitations in identifying novel targets.
Methods: We applied an in situ secretory protein labeling technique using TurboID to analyze secretome from tamoxifen-resistant (TAMR) BC. The increased expression of LGALS3BP was validated using western blotting, qPCR, ELISA, and IF. Chromatin immunoprecipitation was applied to analyze estrogen-dependent regulation of LGALS3BP transcription. The adhesive and angiogenic functions of LGALS3BP were evaluated by abrogating LGALS3BP expression using either shRNA-mediated knockdown or a neutralizing antibody. Xenograft mouse experiments were employed to assess the in vivo metastatic potential of TAMR cells and the LGALS3BP protein. Clinical evaluation of LGALS3BP risk was carried out with refractory clinical specimens from tamoxifen-treated ER-positive BC patients and publicly available databases.
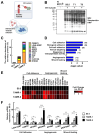
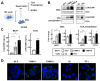
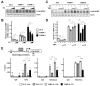
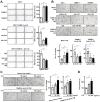
Results: TAMR secretome analysis revealed that 176 proteins were secreted at least 2-fold more from MCF7/TAMR cells than from sensitive cells, and biological processes such as cell adhesion and angiogenesis were associated with the TAMR secretome. Galectin-3 binding protein (LGALS3BP) was one of the top 10 most highly secreted proteins in the TAMR secretome. The expression level of LGALS3BP was suppressed by estrogen signaling, which involves direct ERα binding to its promoter region. Secreted LGALS3BP in the TAMR secretome helped BC cells adhere to the extracellular matrix and promoted the tube formation of human umbilical vein endothelial cells. Compared with sensitive cells, xenograft animal experiments with MCF7/TAMR cells showed increased pulmonary metastasis, which completely disappeared in LGALS3BP-knockdown TAMR cells. Finally, higher levels of LGALS3BP were associated with poor prognosis in ER-positive BC patients treated with adjuvant tamoxifen in the clinic.
Conclusion: TAMR secretome analysis identified secretory proteins, such as LGALS3BP, that are involved in biological processes closely related to metastasis. Secreted LGALS3BP from the TAMR cells promoted adhesion of the cells to the extracellular matrix and vasculature formation, which may support metastasis of TAMR cells.
Keywords: LGALS3BP; Metastasis; Secretome; Tamoxifen-resistant breast cancer.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: The animal experiments were performed in accordance with the guidelines of the Seoul National University Animal Care and Use Committee (SNU-210906-2-3 and SNU-240124-3). Competing interests: The authors declare no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

