Treatment of metastatic urothelial carcinoma in the United Kingdom, France, Germany, Italy, and Spain
- PMID: 39789976
- PMCID: PMC11845109
- DOI: 10.1080/14796694.2024.2445498
Treatment of metastatic urothelial carcinoma in the United Kingdom, France, Germany, Italy, and Spain
Abstract
Introduction: The treatment landscape of metastatic urothelial carcinoma (mUC) has evolved with the emergence of programmed cell death protein 1/ligand 1 (PD-1/L1) inhibitors. This study assessed mUC treatment patterns in Europe.
Methods: Data were derived from the Adelphi mUC Disease Specific Programme™ (November 2020 to April 2021), a large, cross-sectional, patient record-based survey of physicians in France, Germany, Italy, Spain, and the United Kingdom. Patient characteristics, treatment patterns across lines of therapy, and treatment durations were assessed.
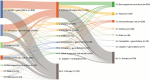
Results: Physicians (N = 232) provided data for 1922 patients with mUC. Mean (SD) patient age at the time of data collection was 69.1 (7.9) years, and 81% presented with bladder tumors. Most patients received platinum-based chemotherapy in first-line (cisplatin plus gemcitabine, 43%; carboplatin plus gemcitabine, 28%), followed by PD-1/L1 inhibitors in second-line (pembrolizumab, 35%; atezolizumab, 19%). In third-line, 41% received best supportive care and 36% received single-agent chemotherapies. Mean treatment duration was longer in second-line than first-line (6.1 vs 4.8 months).
Conclusions: Most patients received platinum-based chemotherapy in first-line, followed by a PD-1/L1 inhibitor. A substantial proportion received best supportive care after second-line. Findings indicate unmet need for the later-line treatment of mUC and provide important context for the emergence of novel therapies.
Keywords: Europe; Metastatic urothelial carcinoma; best supportive care; chemotherapy; enfortumab vedotin; erdafitinib; immune checkpoint inhibitors; real-world evidence.
Plain language summary
What is this article about? This was an international survey of physicians and their patients treated for an advanced form of bladder cancer called metastatic urothelial carcinoma (mUC). The survey collected data to better understand how mUC is treated in Europe.What were the results? Patients with mUC generally received treatments recommended by medical guidelines. Most were first treated with a type of chemotherapy containing platinum. Patients whose disease progressed after their first treatment typically received therapy that affects the immune system to treat cancer. Most patients who needed even further treatment received only palliative care to help maintain their quality of life.What do the results of the study mean? Access to more treatments to improve outcomes for patients with mUC is needed. These results increase our understanding of how mUC is currently being treated, so new treatments can be evaluated comprehensively.
Conflict of interest statement
Acknowledgement
Medical writing and editorial support were provided by Sarah Thompson, PharmD, and Beth Lesher, PharmD, BCPS, from OPEN Health Evidence & Access, Parsippany, NJ, and Sherri Damlo, MS, ELS, from Peloton Advantage, an OPEN Health company, Parsippany, NJ, and were funded by Astellas Pharma Inc. and Pfizer.
Figures
References
-
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. - PubMed
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
