Photochemical Stabilization of Self-Assembled Spherical Nucleic Acids
- PMID: 39790078
- PMCID: PMC11840461
- DOI: 10.1002/smll.202407742
Photochemical Stabilization of Self-Assembled Spherical Nucleic Acids
Abstract
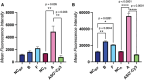
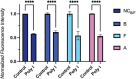
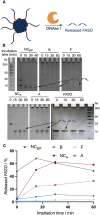
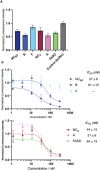
Oligonucleotide therapeutics, including antisense oligonucleotides and small interfering RNA, offer promising avenues for modulating the expression of disease-associated proteins. However, challenges such as nuclease degradation, poor cellular uptake, and unspecific targeting hinder their application. To overcome these obstacles, spherical nucleic acids have emerged as versatile tools for nucleic acid delivery in biomedical applications. Our laboratory has introduced sequence-defined DNA amphiphiles which self-assemble in aqueous solutions. Despite their advantages, self-assembled SNAs can be inherently fragile due to their reliance on non-covalent interactions and fall apart in biologically relevant conditions, specifically by interaction with serum proteins. Herein, this challenge is addressed by introducing two methods of covalent crosslinking of SNAs via UV irradiation. Thymine photodimerization or disulfide crosslinking at the micellar interface enhance SNA stability against human serum albumin binding. This enhanced stability, particularly for disulfide crosslinked SNAs, leads to increased cellular uptake. Furthermore, this crosslinking results in sustained activity and accessibility for release of the therapeutic nucleic acid, along with improvement in unaided gene silencing. The findings demonstrate the efficient stabilization of SNAs through UV crosslinking, influencing their cellular uptake, therapeutic release, and ultimately, gene silencing activity. These studies offer promising avenues for further optimization and exploration of pre-clinical, in vivo studies.
Keywords: crosslinking; delivery; disulfide; gene silencing; oligonucleotides; self‐assembly; spherical nucleic acids.
© 2025 The Author(s). Small published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Samaridou E., Heyes J., Lutwyche P., Adv. Drug Delivery Rev. 2020, 154, 37. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

