Endothelial activating transcription factor 3 promotes angiogenesis and vascular repair in the mouse retina
- PMID: 39790557
- PMCID: PMC11714383
- DOI: 10.1016/j.isci.2024.111516
Endothelial activating transcription factor 3 promotes angiogenesis and vascular repair in the mouse retina
Abstract
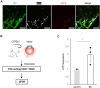
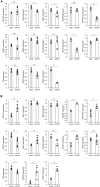
Ischemia and pathological angiogenesis in retinal vascular diseases cause serious vision-related problems. However, the transcriptional regulators of vascular repair remain unidentified. Thus, the factors and mechanisms involved in angiogenesis must be elucidated to develop approaches for restoring normal blood vessels. Here, we investigated the effects of the stress response activating transcription factor 3 (ATF3) on angiogenesis and vascular regeneration in vitro and in vivo. ATF3 was expressed specifically in retinal vascular endothelial cells (ECs) during vascular development. Vascular endothelial growth factor stimulation upregulated ATF3 expression in cultured ECs. The downregulated ATF3 expression in ECs caused the deterioration of vascular network formation in vitro and in vivo. Moreover, ATF3 deletion in a model of oxygen-induced retinopathy inhibited retinal vascular repair but not pathological neovascularization. Transcriptome analysis confirmed that high ATF3 expression upregulated the expression of angiogenesis-related genes in ECs. ATF3 may aid vascular repair therapy in retinal vascular diseases.
Keywords: Ophthalmology; Transcriptomics; Vascular remodeling.
© 2024 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Shukla D., Singh J., Sudheer G., Soman M., John R.K., Ramasamy K., Perumalsamy N. Familial exudative vitreoretinopathy (FEVR). Clinical profile and management. Indian J. Ophthalmol. 2003;51:323–328. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

