Severe neonatal hypotonia due to SLC30A5 variant affecting function of ZnT5 zinc transporter
- PMID: 39790720
- PMCID: PMC11712426
- DOI: 10.1002/jmd2.12465
Severe neonatal hypotonia due to SLC30A5 variant affecting function of ZnT5 zinc transporter
Abstract
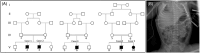
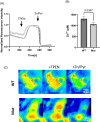
The tightly-regulated spatial and temporal distribution of zinc ion concentrations within cellular compartments is controlled by two groups of Zn2+ transporters: the 14-member ZIP/SLC39 family, facilitating Zn2+ influx into the cytoplasm from the extracellular space or intracellular organelles; and the 10-member ZnT/SLC30 family, mobilizing Zn2+ in the opposite direction. Genetic aberrations in most zinc transporters cause human syndromes. Notably, previous studies demonstrated osteopenia and male-specific cardiac death in mice lacking the ZnT5/SLC30A5 zinc transporter, and suggested association of two homozygous frameshift SLC30A5 variants with perinatal mortality in humans, due to hydrops fetalis and hypertrophic cardiomyopathy. We set out to decipher the molecular basis of a severe hypotonia syndrome. Combining homozygosity mapping and exome sequencing studies of consanguineous Bedouin kindred, as well as transfection experiments and zinc monitoring in HEK293 cells, we demonstrate that a bi-allelic in-frame 3bp deletion variant in SLC30A5, deleting isoleucine within the highly conserved cation efflux domain of the encoded ZnT5, results in lower cytosolic zinc concentrations, causing a syndrome of severe non-progressive neonatal axial and limb hypotonia with high-arched palate and respiratory failure. There was no evidence of hydrops fetalis, cardiomyopathy or multi-organ involvement. Affected infants required nasogastric tube or gastrostomy feeding, suffered from various degrees of respiratory compromise and failure to thrive and died in infancy. Thus, a biallelic variant in SLC30A5 (ZnT5), affecting cytosolic zinc concentrations, causes a severe hypotonia syndrome with respiratory insufficiency and failure to thrive, lethal by 1 year of age.
Keywords: SLC30A5; ZnT5; hypotonia; mutation; neurological syndrome.
© 2024 The Author(s). JIMD Reports published by John Wiley & Sons Ltd on behalf of SSIEM.
Conflict of interest statement
Vadim Dolgin, Pauline Chabosseau, Jacob Bistritzer, Iris Noyman, Orna Staretz‐Chacham, Ohad Wormser, Noam Hadar, Marina Eskin‐Schwartz, Bibi Kanengisser‐Pines, Ginat Narkis, Ramy Abramsky, Eilon Shany, Guy A. Rutter, Kyla Marks, and Ohad S. Birk declare that they have no conflict of interest. Guy A. Rutter has received grant funding from, and is a consultant for, Sun Pharmaceuticals Industries Ltd. This company was not involved in any way in the design or execution of the present study.
Figures
References
LinkOut - more resources
Full Text Sources

