Impaired cerebral microvascular reactivity and endothelial SK channel activity in a streptozotocin-treated mouse model of Alzheimer's disease
- PMID: 39791382
- PMCID: PMC11892793
- DOI: 10.1177/13872877241309120
Impaired cerebral microvascular reactivity and endothelial SK channel activity in a streptozotocin-treated mouse model of Alzheimer's disease
Abstract
Background: Alzheimer's disease (AD) is a complex neurodegenerative disease marked by increased amyloid-β (Aβ) deposition, tau hyperphosphorylation, impaired energy metabolism, and chronic ischemia-type injury. Cerebral microvascular dysfunction likely contributes to AD pathology, but its precise pathogenic role has been poorly defined.
Objective: To examine microvascular reactivity to endothelium-dependent vasodilators and small conductance calcium-activated potassium (SK) channel activity in an intracerebral streptozotocin (STZ)-induced AD mouse model.
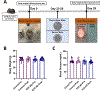
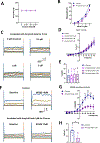
Methods: Control and STZ-AD mice underwent Morris Water Maze and Barnes testing, after which cerebral microvascular and brain microvascular endothelial cells (MBMECs) were dissected to assess microvascular reactivity, responses to SK channel activator NS309, and ion-channel current recordings using whole-cell patch clamp methodology. Control mouse cerebral microvascular and human brain microvascular endothelial cells (HBMECs) were treated with soluble Aβ1-42 peptide to characterize microvascular reactivity and endothelial potassium currents.
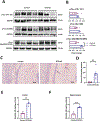
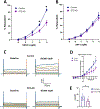
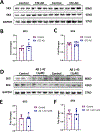
Results: STZ-AD mice exhibited impaired performance vs control mice in behavioral testing. STZ-AD mice also exhibited diminished cerebral microvascular responsiveness and MBMECs potassium current augmentation in response to NS309 compared with control mice. Incubation of control mouse cerebral micro-vessels and HBMECs with soluble Aβ (1 µM) for 2 h attenuated relaxation responses to NS309 and diminished NS309-sensitive endothelial potassium currents.
Conclusions: STZ-AD mice exhibited impaired microvascular relaxation responses to endothelium-dependent vasodilators; SK/IK channel dysfunction may be involved in the mechanism of this impairment. Acute treatment with Aβ produced dysregulated cerebrovascular endothelial SK/IK channels. Further elucidation of the role of microvascular dysfunction in AD is needed to prevent the chronic ischemia-type injury that contributes to cognitive decline.
Keywords: Alzheimer's disease; Morris water maze; NS309; SK channels; amyloid-β; cerebral endothelial dysfunction; cerebral microvascular dysfunction; streptozotocin.
Conflict of interest statement
Declaration of conflicting interestsThe authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

References
-
- Alzheimer’s disease facts and figures. Alzheimer’s Disease and Dementia. https://www.alz.org/alzheimers-dementia/facts-figures#prevalence (accessed 18 March 2023)
-
- Wong W Economic burden of Alzheimer disease and managed care considerations. Am J Manag Care 2020; 26: S177–S183. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

