Ezrin Polarization as a Diagnostic Marker for Circulating Tumor Cells in Hepatocellular Carcinoma
- PMID: 39791707
- PMCID: PMC11720075
- DOI: 10.3390/cells14010006
Ezrin Polarization as a Diagnostic Marker for Circulating Tumor Cells in Hepatocellular Carcinoma
Abstract
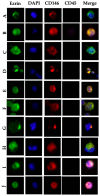
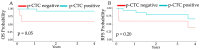
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third leading cause of cancer-related death worldwide, with no precise method for early detection. Circulating tumor cells (CTCs) expressing the dynamic polarity of the cytoskeletal membrane protein, ezrin, have been proposed to play a crucial role in tumor progression and metastasis. This study investigated the diagnostic and prognostic potential of polarized circulating tumor cells (p-CTCs) in HCC patients. CTCs were isolated from the peripheral blood of 20 HCC patients and 18 patients with nonmalignant liver disease (NMLD) via an OncoQuick® kit and immunostained with Ezrin-Alexa Fluor 488®, CD146-PE, and CD45-APC. A fluorescence microscopy was then performed for analysis. The HCC group exhibited significantly higher levels of p-CTCs, with median values of 0.56 p-CTCs/mL, compared to 0.02 p-CTCs/mL (p = 0.03) in the NMLD group. CTCs were detected in 95% of the HCC patients, with a sensitivity of 95% and specificity of 89%. p-CTCs were present in 75% of the HCC patients, with a sensitivity of 75% and a specificity of 94%. Higher p-CTC counts were associated with the significantly longer overall survival in HCC patients (p = 0.05). These findings suggest that p-CTCs could serve as valuable diagnostic and prognostic markers for HCC. The incorporation of p-CTCs into diagnostic strategies could enhance therapeutic decision-making and improve patient outcomes.
Keywords: cancer; circulating tumor cells; ezrin; hepatocellular carcinoma; liver surgery; personalized diagnosis and therapy; polarization; single-cell polarity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

