Unravelling Secondary Brain Injury: Insights from a Human-Sized Porcine Model of Acute Subdural Haematoma
- PMID: 39791718
- PMCID: PMC11720468
- DOI: 10.3390/cells14010017
Unravelling Secondary Brain Injury: Insights from a Human-Sized Porcine Model of Acute Subdural Haematoma
Abstract
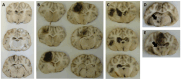
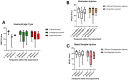
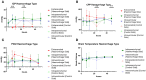
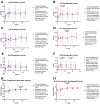
Traumatic brain injury (TBI) remains one of the leading causes of death. Because of the individual nature of the trauma (brain, circumstances and forces), humans experience individual TBIs. This makes it difficult to generalise therapies. Clinical management issues such as whether intracranial pressure (ICP), cerebral perfusion pressure (CPP) or decompressive craniectomy improve patient outcome remain partly unanswered. Experimental drug approaches for the treatment of secondary brain injury (SBI) have not found clinical application. The complex, cellular and molecular pathways of SBI remain incompletely understood, and there are insufficient experimental (animal) models that reflect the pathophysiology of human TBI to develop translational therapeutic approaches. Therefore, we investigated different injury patterns after acute subdural hematoma (ASDH) as TBI in a post-hoc approach to assess the impact on SBI in a long-term, human-sized porcine TBI animal model. Post-mortem brain tissue analysis, after ASDH, bilateral ICP, CPP, cerebral oxygenation and temperature monitoring, and biomarker analysis were performed. Extracerebral, intraparenchymal-extraventricular and intraventricular blood, combined with brainstem and basal ganglia injury, influenced the experiment and its outcome. Basal ganglia injury affects the duration of the experiment. Recognition of these different injury patterns is important for translational interpretation of results in this animal model of SBI after TBI.
Keywords: acute subdural haematoma; animal model; guidelines; intracranial pressure; management; multimodal brain monitoring; outcome; secondary brain injury; traumatic brain injury.
Conflict of interest statement
Author Claire Hartmann conducted all experiments as an employed physician of the University Hospital Ulm. After finishing the series of experiment, she was employed by the company Boehringer Ingelheim Pharma. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The Boehringer Ingelheim Pharma had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures





References
-
- Maas A.I.R., Menon D.K., Manley G.T., Abrams M., Akerlund C., Andelic N., Aries M., Bashford T., Bell M.J., Bodien Y.G., et al. Traumatic brain injury: Progress and challenges in prevention, clinical care, and research. Lancet Neurol. 2022;21:1004–1060. doi: 10.1016/S1474-4422(22)00309-X. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

