Identification of B Cell Subpopulations with Pro- and Anti-Tumorigenic Properties in an Immunocompetent Mouse Model of Head and Neck Squamous Cell Carcinoma
- PMID: 39791721
- PMCID: PMC11720715
- DOI: 10.3390/cells14010020
Identification of B Cell Subpopulations with Pro- and Anti-Tumorigenic Properties in an Immunocompetent Mouse Model of Head and Neck Squamous Cell Carcinoma
Abstract
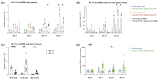
Due to their high developmental diversity and different regulatory and functional roles, B cell subpopulations can promote or inhibit tumor growth. An orthotopic murine HNSCC model was applied to investigate the B cell composition and function in HNSCCs. Using flow cytometry approaches, cells from the spleen, lymph nodes and tumors were analyzed. Additionally, immunoglobulin (Ig) levels post-tumor induction were tracked via enzyme-linked immunosorbent assays (ELISA). Following tumor induction, GCs, as well as increasing numbers of GL7+CD95+ GC B cells in the spleen and tumor tissues, were detected. In parallel, we observed CD39+CD73+ B cells in tumors and spleens of tumor-bearing mice. Notably, CD39+CD73+ expression was primarily detected on MZ B cells and to a lesser extent on follicular (FO) and non-follicular, newly formed (NF) B cells, supposing an immunosuppressive function of MZ B cells in the TME. Parallel to increased MZ B cell numbers in secondary lymphoid organs (SLOs) as well as in the tumor tissue, IgM antibody (Ab) levels rose continuously. In contrast, IgG1, IgG2, and IgG3 levels increased at later time points. Understanding the complex interactions between B cell subsets and the TME could lead to new strategies for enhancing the treatment and prognosis of HNSCC patients.
Keywords: B cells; HNSCC; adenosine; germinal centers; immunocompetent mouse model.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

