Dysregulation of Podocyte BK Channels and Nephrosis: Effects of Circulating Factors and Auxiliary β4 Subunits
- PMID: 39791723
- PMCID: PMC11719602
- DOI: 10.3390/cells14010022
Dysregulation of Podocyte BK Channels and Nephrosis: Effects of Circulating Factors and Auxiliary β4 Subunits
Abstract
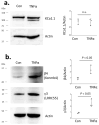
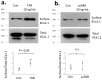
Podocytes express large-conductance Ca2+-activated K+ channels (BK channels) and at least two different pore-forming KCa1.1 subunit C-terminal splice variants, known as VEDEC and EMVYR, along with auxiliary β and γ subunits. Podocyte KCa1.1 subunits interact directly with TRPC6 channels and BK channels become active in response to Ca2+ influx through TRPC6. Here, we confirmed that Ca2+ influx through TRPC channels is reduced following the blockade of BK channels by paxilline. The overall abundance of KCa1.1 subunits, as well as that of β4 and γ3 subunits, were increased in glomeruli isolated from Sprague Dawley rats during chronic puromycin aminonucleoside (PAN) nephrosis. Exposing cultured mouse podocytes for 24 h to recombinant TNFα, a circulating factor implicated in pediatric nephrotic syndromes, did not affect the total abundance of KCa1.1, but did evoke significant increases in both β4 and γ3. However, TNFα evoked a marked increase in the surface abundance of KCa1.1 subunits, similar to that of its previously reported effects on TRPC6 channels. The effect of TNFα on the surface expression of KCa1.1 was eliminated following siRNA knockdown of the β4 subunits, suggesting a role for this subunit in KCa1.1 trafficking to the cell surface. By contrast, treating podocytes with suPAR did not affect the total or surface expression of KCa1.1. The coordinated activation of KCa1.1 channels may promote Ca2+ influx through TRPC channels during normal and abnormal podocyte function by maintaining a membrane potential that allows for the efficient permeation of divalent cations through TRPC pores.
Keywords: BK channel; TNFα; TRPC6; calcium; circulating factor; nephrosis; podocyte.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





Similar articles
-
Canonical transient receptor potential channel (TRPC)3 and TRPC6 associate with large-conductance Ca2+-activated K+ (BKCa) channels: role in BKCa trafficking to the surface of cultured podocytes.Mol Pharmacol. 2009 Mar;75(3):466-77. doi: 10.1124/mol.108.051912. Epub 2008 Dec 3. Mol Pharmacol. 2009. PMID: 19052171 Free PMC article.
-
Syndecan-4 ectodomain evokes mobilization of podocyte TRPC6 channels and their associated pathways: An essential role for integrin signaling.Biochim Biophys Acta. 2015 Oct;1853(10 Pt A):2610-20. doi: 10.1016/j.bbamcr.2015.07.011. Epub 2015 Jul 17. Biochim Biophys Acta. 2015. PMID: 26193076
-
TRPC6 interacted with KCa1.1 channels to regulate the proliferation and apoptosis of glioma cells.Arch Biochem Biophys. 2022 Aug 15;725:109268. doi: 10.1016/j.abb.2022.109268. Epub 2022 Apr 28. Arch Biochem Biophys. 2022. PMID: 35489424
-
Regulation of BK Channels by Beta and Gamma Subunits.Annu Rev Physiol. 2019 Feb 10;81:113-137. doi: 10.1146/annurev-physiol-022516-034038. Annu Rev Physiol. 2019. PMID: 30742788 Free PMC article. Review.
-
Modulation of BK Channel Function by Auxiliary Beta and Gamma Subunits.Int Rev Neurobiol. 2016;128:51-90. doi: 10.1016/bs.irn.2016.03.015. Epub 2016 Apr 8. Int Rev Neurobiol. 2016. PMID: 27238261 Free PMC article. Review.
Cited by
-
Modulation of podocyte extracellular matrix remodeling in membranous nephropathy by the NFATc3/LRRC55/BK channel pathway.J Cell Commun Signal. 2025 Jun 13;19(2):e70022. doi: 10.1002/ccs3.70022. eCollection 2025 Jun. J Cell Commun Signal. 2025. PMID: 40520074 Free PMC article.
References
-
- Winn M.P., Conlon P.J., Lynn K.L., Farrington M.K., Creazzo T., Hawkins A.F., Daskalakis N., Kwan S.Y., Ebersviller S., Burchette J.L., et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005;308:1801–1804. doi: 10.1126/science.1106215. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

