Mechanisms of Rhodopsin-Related Inherited Retinal Degeneration and Pharmacological Treatment Strategies
- PMID: 39791750
- PMCID: PMC11720364
- DOI: 10.3390/cells14010049
Mechanisms of Rhodopsin-Related Inherited Retinal Degeneration and Pharmacological Treatment Strategies
Abstract
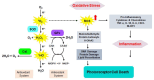
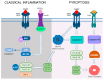
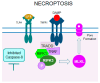
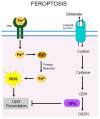
Retinitis pigmentosa (RP) is a hereditary disease characterized by progressive vision loss ultimately leading to blindness. This condition is initiated by mutations in genes expressed in retinal cells, resulting in the degeneration of rod photoreceptors, which is subsequently followed by the loss of cone photoreceptors. Mutations in various genes expressed in the retina are associated with RP. Among them, mutations in the rhodopsin gene (RHO) are the most common cause of this condition. Due to the involvement of numerous genes and multiple mutations in a single gene, RP is a highly heterogeneous disease making the development of effective treatments particularly challenging. The progression of this disease involves complex cellular responses to restore cellular homeostasis, including the unfolded protein response (UPR) signaling, autophagy, and various cell death pathways. These mechanisms, however, often fail to prevent photoreceptor cell degradation and instead contribute to cell death under certain conditions. Current research focuses on the pharmacological modulation of the components of these pathways and the direct stabilization of mutated receptors as potential treatment strategies. Despite these efforts, the intricate interplay between these mechanisms and the diverse causative mutations involved has hindered the development of effective treatments. Advancing our understanding of the interactions between photoreceptor cell death mechanisms and the specific genetic mutations driving RP is critical to accelerate the discovery and development of therapeutic strategies for this currently incurable disease.
Keywords: misfolding; neuroinflammation; oxidative stress; photoreceptor; retinal degeneration; rhodopsin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Kolb H. Simple Anatomy of the Retina. In: Kolb H., Fernandez E., Jones B., Nelson R., editors. Webvision: The Organization of the Retina and Visual System. University of Utah Health Sciences Center; Salt Lake City, UT, USA: 1995.
-
- Ramirez A.I., de Hoz R., Salobrar-Garcia E., Salazar J.J., Rojas B., Ajoy D., Lopez-Cuenca I., Rojas P., Trivino A., Ramirez J.M. The Role of Microglia in Retinal Neurodegeneration: Alzheimer’s Disease, Parkinson, and Glaucoma. Front. Aging Neurosci. 2017;9:214. doi: 10.3389/fnagi.2017.00214. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

