Small Extracellular Vesicles Promote Axon Outgrowth by Engaging the Wnt-Planar Cell Polarity Pathway
- PMID: 39791757
- PMCID: PMC11720052
- DOI: 10.3390/cells14010056
Small Extracellular Vesicles Promote Axon Outgrowth by Engaging the Wnt-Planar Cell Polarity Pathway
Abstract
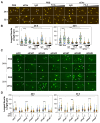
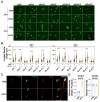
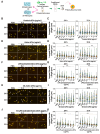
In neurons, the acquisition of a polarized morphology is achieved upon the outgrowth of a single axon from one of several neurites. Small extracellular vesicles (sEVs), such as exosomes, from diverse sources are known to promote neurite outgrowth and thus may have therapeutic potential. However, the effect of fibroblast-derived exosomes on axon elongation in neurons of the central nervous system under growth-permissive conditions remains unclear. Here, we show that fibroblast-derived sEVs promote axon outgrowth and a polarized neuronal morphology in mouse primary embryonic cortical neurons. Mechanistically, we demonstrate that the sEV-induced increase in axon outgrowth requires endogenous Wnts and core PCP components including Prickle, Vangl, Frizzled, and Dishevelled. We demonstrate that sEVs are internalized by neurons, colocalize with Wnt7b, and induce relocalization of Vangl2 to the distal axon during axon outgrowth. In contrast, sEVs derived from neurons or astrocytes do not promote axon outgrowth, while sEVs from activated astrocytes inhibit elongation. Thus, our data reveal that fibroblast-derived sEVs promote axon elongation through the Wnt-PCP pathway in a manner that is dependent on endogenous Wnts.
Keywords: axon outgrowth; cortical neurons; extracellular vesicles; neurite outgrowth; planar cell polarity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

