Substructure-Specific Antibodies Against Fentanyl Derivatives
- PMID: 39792034
- PMCID: PMC11781026
- DOI: 10.1021/acsnano.4c14369
Substructure-Specific Antibodies Against Fentanyl Derivatives
Abstract
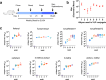
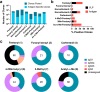
Structural variants of the synthetic opioid fentanyl are a major threat to public health. Following an investigation showing that many derivatives are poorly detected by commercial lateral flow and related assays, we created hapten conjugate vaccines using an immunogenic virus-like particle carrier and eight synthetic fentanyl derivatives designed to mimic the structural features of several of the more dangerous analogues. Immunization of mice elicited strong antihapten humoral responses, allowing the screening of hundreds of hapten-specific hybridomas for binding strength and specificity. A panel of 13 monoclonal IgG antibodies were selected, each showing a different pattern of recognition of fentanyl structural variations, and all proving to be highly efficient at capturing parent fentanyl compounds in competition ELISA experiments. These results provide antibody reagents for assay development as well as a demonstration of the power of the immune system to create binding agents capable of both broad and specific recognition of small-molecule targets.
Keywords: antibodies; diagnostics; fentanyl derivatives; immune response; immunization; virus-like particles.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Spencer M. R. M. A. M.; Warner M.. Drug Overdose Deaths in the United States, 2001–2021. NCHS Data Brief, no. 457; National Center for Health Statistics: Hyattsville, MD, 2022.
-
- Centers for Disease Control and Prevention . State Unintentional Drug Overdose Reporting System (SUDORS). Final Data. US Department of Health and Human Services, CDC, Atlanta, GA; October 30. 2023. access at: https://www.cdc.gov/drugoverdose/fatal/dashboard.
-
- Van Bever W. F.; Niemegeers C.; Schellekens K.; Janssen P. N-4-Substituted 1-(2-arylethyl)-4-piperidinyl-N-phenylpropanamides, a novel series of extremely potent analgesics with unusually high safety margin. Arzneim.-Forsch. 1976, 26, 1548–1551. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

