Anti-Syndecan 2 Antibody Treatment Reduces Edema Formation and Inflammation of Murine Laser-Induced CNV
- PMID: 39792057
- PMCID: PMC11730891
- DOI: 10.1167/tvst.14.1.10
Anti-Syndecan 2 Antibody Treatment Reduces Edema Formation and Inflammation of Murine Laser-Induced CNV
Abstract
Purpose: Alteration of visual acuity in wet age-related macular degeneration (AMD) is mostly driven by vascular endothelial growth factor A (VEGF-A)-induced edema from leaky newly forming blood vessels below the retina layers. To date, all therapies aimed at alleviation of this process have relied on inhibition of VEGF-A activity. Although effective in preventing vascular leak and edema, this approach also leads to the loss of normal vasculature and multiple related side effects.
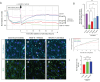
Methods: We have developed an alternative strategy that uses anti-syndecan-2 polyclonal antibody (anti-Sdc2 pAb) to block VEGF-A-induced permeability without interfering with other VEGF-A activities. The effect of anti-Sdc2 pAb therapy was assessed in vitro using a transendothelial electrical resistance (TEER) assay, as well as staining of the endothelial cell junction, and in vivo in the laser-induced choroidal neovascularization (CNV) model.
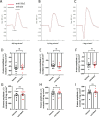
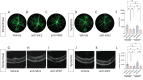
Results: Anti-Sdc2 pAb blocked VEGF-A-induced permeability in vitro, and both local intravitreal injections and systemic intravenous treatments with anti-Sdc2 pAb were as effective as intravitreal anti-VEGF therapy in reducing edema, size of retinal lesions, and local inflammation in this model. Post-injury neovascularization was not affected by treatment with anti-Sdc2 pAb.
Conclusions: These findings indicate that anti-Sdc2 pAb therapy can be an effective alternative to anti-VEGF-A approaches for suppression of edema and to prevent retinal lesions in wet neovascular AMD (nAMD).
Translational relevance: Intravitreal anti-Sdc2 treatment may avoid side effects observed with the long-term anti-VEGF therapy, and systemic treatment with an anti-Sdc2 pAb antibody can address the issues associated with repeated intravitreal injections.
Conflict of interest statement
Disclosure:
Figures

References
-
- Wong WL, Su X, Li X, et al. .. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014; 2: e106–e116. - PubMed
-
- Keenan TDL, Cukras CA, Chew EY.. Age-related macular degeneration: epidemiology and clinical aspects. Adv Exp Med Biol. 2021; 1256: 1–31. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

